Health Canada 2021–22 Departmental Results Report
Table of contents
- From the Ministers
- Results at a glance
- Results: what we achieved
- Sex- and Gender-Based Analysis Plus
- Spending and human resources
- Corporate information
- Supporting information on the program inventory
- Supplementary information tables
- Federal tax expenditures
- Organizational contact information
- Appendix: definitions
- Footnotes
From the Ministers
This year's Departmental Results Report reflects an overview of Health Canada's continued efforts to support a comprehensive response to COVID-19, while also delivering on its core work of protecting the health and safety of Canadians.
In 2021-22, Health Canada and the Public Health Agency of Canada worked with other federal partners, as well as jurisdictions and stakeholders across the country to implement a successful vaccination campaign against COVID-19. Over the past year, by expediting the regulatory review of COVID-19 health products without compromising safety, efficacy and quality standards, Health Canada authorized new and supplemental submissions for COVID-19 vaccines (including boosters), therapeutics and test kits, to help protect Canadians against the impacts of COVID. The Department continued to monitor the use of these health products once they were on the market to ensure they met its stringent quality standards.
Health Canada also took important steps towards strengthening Canada's capacity to develop and produce made-in-Canada vaccines. This included providing expert guidance to pharmaceutical companies who are developing or entering the Canadian biomanufacturing market.
Testing is a critical tool in slowing virus transmission, in concert with public health measures. Health Canada worked with the Public Health Agency of Canada and partner departments to procure and distribute over 402 million rapid tests to Canadians via provinces and territories, and through distribution channels to private and public sector organizations for workplace screening. Through Safe Restart Agreements, Health Canada provided testing guidance and financial support to provinces and territories, including health human resources and other surge supports to healthcare systems under stress; supported testing and wastewater surveillance research and infrastructure; as well as supported strengthening data management for P/Ts and Indigenous organizations.
With overdose deaths driven by a highly unpredictable and toxic illegal drug supply, the pandemic continued to have devastating effects on people who use substances, their loved ones and their communities. Public health restrictions compounded risks for people who use drugs by reducing access to treatment and harm reduction services. In response, the Department reinforced frontline services by funding 180 community-based substance use projects that supported those most in need and saved lives by reducing the stigma and the harm of substance use.
Health Canada also championed greater access to, and knowledge of, pharmaceutical-grade medications as an alternative to the toxic illegal drug supply (safer supply) as an effective intervention to help reduce overdose deaths. The Department funded 18 safer supply projects across the country, including a National Safer Supply Community of Practice.
Health Canada collaborated with provinces and territories to integrate virtual care into health services, and leveraged digital health tools and data to improve the health system and outcomes for Canadians. Investments in virtual care initiatives and digital infrastructure helped ensure access to essential health services during the pandemic, particularly in rural and remote areas.
The Safe Long-term Care Fund strengthened support to jurisdictions in response to the increasing challenges faced by the long-term care system. It funded efforts to strengthen infection prevention and control, as well as the development of standards that aim to ensure safe, high quality, accessible long-term care across the country.
Of course, Canadians rely on our health system for health issues beyond COVID-19. Health Canada continued to uphold universality as a pillar of Canada's health care system, but also supported and encouraged sustainable and adaptable health care systems that ensure access for all Canadians to appropriate and effective health care services they deserve.
As part of this work, this past year, Health Canada signed an agreement with the Standards Council of Canada to develop standards for mental health and substance use services. This will help formalize what Canadians can expect in terms of the timeliness and quality of these services. At the same time, the Wellness Together Canada portal and its companion app, Pocketwell, continued to provide easily accessible mental health support and resources.
We also made progress toward a national pharmacare program that will improve access to and affordability of drugs for Canadians by establishing the Canadian Drug Agency Transition Office. In addition, we worked closely with partners to develop a national strategy for drugs for rare diseases, to help affected Canadians access drugs that could help manage and treat their conditions.
Protecting Canadians from unsafe substances and consumer products also remained an important focus over the past year. Health Canada continued to work with jurisdictions and stakeholders to mitigate youth vaping by limiting the nicotine concentration and restricting flavours in vaping products to reduce their appeal.
Along the same theme of protecting Canadians from unsafe products, the Government of Canada worked toward protecting the environment from the risks associated with pesticide use by establishing a Transformation Task Force to renew the pesticide regulatory program. This program is part of a government-wide initiative to create a more transparent pesticide review process.
Health Canada continued to support inclusive healthcare initiatives for members of 2SLGBTQI+ communities, Indigenous and racialized people, persons with disabilities, women, and youth. In 2021-22, the Department funded projects aimed at closing gaps in access to support and services for sexual and reproductive health care for these higher risk and often-marginalized populations.
We would like to close by acknowledging Health Canada's steadfast, professional employees, who have persevered through yet another challenging year to deliver outstanding programs and services to Canadians. We are proud of the results we have achieved together and look forward to continue building a health system that will serve Canadians now, and into the future.
The Honourable Jean-Yves Duclos, P.C. M.P.
Minister of Health
The Honourable Dr. Carolyn Bennett, P.C. M.P.
Minister of Mental Health and Addictions and Associate Minister of Health
Results at a glance
Resources used to achieve results for Canadians
Health Canada's total actual spending for 2021-22: $6,044,784,701
Health Canada's total actual full-time equivalents for 2021-22: 9,528
Health Canada is the federal department responsible for helping Canadians maintain and improve their health. In keeping with the Department's commitment to making this country's population among the healthiest in the world, its main responsibilities are as a regulator, a catalyst for innovation, a funder, and an information provider.
Health Canada also administers the Canada Health Act (CHA), which embodies national principles to ensure a universal and equitable publicly-funded health care system. In addition to working closely with provincial and territorial (P/T) governments, the Department also works with partners in the Health Portfolio (Public Health Agency of Canada [PHAC], Canadian Food Inspection Agency [CFIA], and Canadian Institutes of Health Research [CIHR]), other federal departments and agencies, non-governmental organizations, other countries, Indigenous partnersFootnote 1 and the private sector.
From coast to coast to coast, Health Canada employees—scientists and researchers, inspectors, doctors and nurses, policy analysts and administrative professionals, and many others—are working to help Canadians maintain and improve their health.
Core Responsibilities
Health Canada's Departmental Results Framework outlines two core responsibilities for the Department: Health Care Systems and Health Protection and Promotion. This reporting framework provides the structure for planned activities, which are organized according to these core responsibilities and their corresponding results
Core Responsibility 1:
Health Care Systems
Core Responsibility 2:
Health Protection and Promotion
Under the Health Care Systems core responsibility, Health Canada provides national leadership to foster sustainable health care systems that ensure access for Canadians to appropriate and effective health care. This is mainly achieved through partnerships with P/T governments and support through targeted funding agreements to organizations and key pan-Canadian health partners that are contributing to health system improvements.
Within the Health Protection and Promotion core responsibility, Health Canada works with domestic and international partners to assess, manage and communicate the health and safety risks and benefits associated with health and consumer products, food, chemicals, pesticides, environmental factors, tobacco and vaping products, cannabis, and controlled substances. These risks are managed through rigorous regulatory frameworks and by communicating risks and benefits to Canadians so that they can make informed decisions.
Key Results
In 2021 - 22, among the many others detailed in this report, Health Canada achieved the following key results that contribute to the health of Canadians.
Core Responsibility 1: Health Care Systems
- Working closely with PHAC, federal partners and other stakeholders, Health Canada continued to address the health impacts of the pandemic and its evolution. The Health Portfolio ensured sufficient domestic supply of COVID-19 vaccines, therapeutics and rapid tests; supported P/Ts via multiple initiatives, such as coping with health system surge capacity through the sourcing of health human resources; and supported international efforts to ensure access to health interventions to fight COVID-19.
- The Department conducted research, analysis and policy work on the following priority health care systems issues: working with PHAC and federal partners to lead Canada's response to COVID-19; integrating virtual care and mental health; health expenditures and funding; primary, home and palliative care; access to sexual and reproductive health services; accessibility and affordability of pharmaceuticals; health human resources and the impacts of health care systems modernization on the health workforce; quality of care; digital health and health data; health care systems and service delivery innovation; as well as health technology.
- Health Canada supported multiple pan-Canadian health organizations that directly contribute to health system improvements in areas such as: digital health; health information; drugs and technologies; mental health and substance use; cancer prevention and control; patient safety and quality of care; and service delivery innovation. By leveraging technology, these investments facilitated access to virtual tools and services during the COVID-19 pandemic and beyond.
- To ensure that Canadians have access to appropriate and effective health services, the Department focused on: expanding access to mental health and substance use services; improving access to quality home, community, long-term and palliative care; supporting primary and virtual care and creating a world-class health data system; supporting testing capacity and developing innovative approaches to testing, screening and research; working towards national universal pharmacare; supporting implementation of medical assistance in dying; combatting cancer; supporting organ, tissue and blood donation and transplantation; supporting access to health services for specific populations.
- In collaboration with P/Ts, health organizations, key stakeholders and Standards Council of Canada, work began towards developing an integrated suite of standards for mental health and substance use services, with a focus on: integration of mental health and substance use in primary care; digital mental health and substance use apps; integrated youth services; substance use treatment facilities; substance use workforce; and mental health and substance use services for complex health needs. At the same time, Health Canada's Wellness Together Canada portal continued to provide Canadians with free access to live and confidential online mental health and substance use resources.
Core Responsibility 2: Health Protection and Promotion
- Health Canada continued to advance the Regulatory Innovation Agenda, a multi-year regulatory modernization plan designed to make the federal regulatory framework more agile and responsive to an innovative environment, while ensuring the system remains science and safety-based.
- The Department worked to ensure that Canadians have access to safe, effective and quality health products by: providing ongoing access to COVID-related health products; promoting timely access to other health products; managing and monitoring drug and medical device shortages; modernizing the way we provide access to drugs not readily available; applying real-world evidence to support regulatory decision-making; strengthening regulatory oversight; modernizing compliance and enforcement; acting to prevent and control antimicrobial resistance; fostering international collaboration and coordination; as well as promoting access to new and emerging technologies.
- Health Canada protected Canadians from unsafe consumer and commercial products and substances by: applying a comprehensive approach to substance use-related harms; regulating cannabis; managing the health risks of chemicals in the home, the workplace and the environment; supporting the safety of consumer products and cosmetics; protecting Canadians from radiation; as well as strengthening pesticide regulation and transparency.
- The Department reinforced its work to provide evidence-informed policy and regulatory direction related to substance use and addiction, monitor substance use, including alcohol, support harm reduction initiatives, and modernize the policies and operational procedures for supervised consumption sites and services. Among other initiatives, it continued to update the Canadian Drugs and Substances Strategy; established an Expert Task Force on Substance Use; funded numerous safer supply projects across the country; and funded a suite of important projects via the Canadian Centre on Substance Use and Addiction.
- Health Canada supported Canadians to make healthy choices in their day-to-day lives, by: promoting healthy eating; improving food packaging and labelling; ensuring the safety of the Canadian food supply; fostering international collaboration and coordination; taking action on youth vaping and reducing tobacco use; as well as supporting Canadians in making informed decisions about cannabis use through public education, research and surveillance.
Internal Services
- In 2021-22, Health Canada furthered its commitment to ensuring a workplace that is free of racism and discrimination, and where all employees feel safe and are treated with respect, dignity and fairness by advancing its Multi-Year Diversity and Employment Equity Plan. The Department's toolkit included the Leadership Council on Diversity, the Mentorship Plus initiative, the Equitable Access to Language Training Program, and the Workplace Accessibility Passport.
- The Department focused its internal activities on: attracting and retaining a diverse, inclusive, and bilingual workforce within a healthy, accessible and high performing workplace; regularly engaging employees and seeking feedback to help protect their mental and physical health and support their work and productivity; modernizing the workplace to enable a safe and productive workforce with access to modern tools and facilities; as well as communications services that continue to engage Canadians with timely and relevant information they needed to take action on their personal and collective health and safety.
Experimentation
Health Canada advanced the creative capacity for its employees by promoting its Innovation and Experimentation Policy Framework. The Department's Solutions Fund continued to provide a space for employees to test ideas and generate evidence to inform decision-making, and this report describes no fewer than 16 funded projects. Under the Innovative Solutions Canada program, Health Canada invested in 5 Canadian companies to develop a prototype of their innovation in response to 3 important health system challenges. Health Canada also collaborated with the National Research Council and Environment and Climate Change Canada to support 2 projects related to the Innovative Solutions Canada COVID-19 challenges.
Sex- and Gender-Based Analysis Plus (SGBA Plus/GBA Plus)
Health Canada renewed both its Sex- and Gender-Based Analysis (SGBA Plus) Policy and associated Action Plan for another 4 years starting in early 2022. The Plan and Policy aim to strengthen the integration of sex, gender and other identity factors (such as age, race and income level) in the externally and internally facing work of the Department and serve as the main driver for advancing equity and improving approaches to diversity and inclusion. Each branch identified at least one signature initiative. This report details progress in the areas of: increasing governance and accountability; strengthening knowledge and capacity; as well as implementing SGBA Plus across Health Canada programs.
For more information on Health Canada's plans, priorities and results achieved, see the “Results: what we achieved” section of this report.
Results: what we achieved
Core Responsibility 1: Health Care Systems
Description
Health Canada provides national leadership to support and encourage sustainable and adaptable health care systems that ensures access for Canadians to appropriate and effective health care services.
The United Nations 2030 Agenda for Sustainable Development and UN Sustainable Development Goals (SDGs)
Health Canada's results under Core Responsibility 1: Health Care Systems directly supported Canada's efforts to address the UN 2030 Agenda, particularly Sustainable Development Goal (SDG) 3, "promoting the good health and well-being of Canadians".
The Department promoted health care system and service delivery innovation, including e-prescribing and access to virtual care; improving patient safety and quality care; and strengthening Canada's health care systems with a focus on improving the capacity to protect vulnerable populations and high-risk communities. For example:
- Federal investments supported virtual care services enabling Canadians, especially marginalized communities, to safely engage with their regular health providers.
Health Canada also improved access to appropriate and effective health care services (including COVID-19 vaccines and treatments; medical assistance in dying; home, community and palliative care; mental health and substance use services; and cancer care); supported health human resources; and continued efforts to create a national, universal system of pharmacare. For example:
- Health Canada supported the ongoing response to address the direct and indirect health impacts of COVID-19.
- The Wellness Together Canada portal, provides free 24/7 access to mental health and substance use resources and supports for all individuals living in Canada, supporting populations who have barriers to care, including those in isolated or remote areas, facing stigma or financial difficulties, and official language minority communities. This supports the Canadian Indicator Framework (CIF) Indicators 3.7.1 and 3.12.1.
All of the above examples supported the CIF Ambition "Canadians Have Healthy and Satisfying Lives".
Results
Departmental Result 1: Canadians have modern and sustainable health care systems
Canadians face an array of complex health concerns, even more so during a pandemic. For over half a century, the Canadian health care system has been strong and reliable in supporting social and economic security. Many Canadians require additional help in dealing with challenging health issues—whether coping with the COVID-19 pandemic, addressing substance use, accessing sexual and reproductive health, or navigating the complexities of end-of-life care. In 2021-22, Health Canada strengthened its close collaboration with provinces and territories (P/Ts), providing them with the policy and financial support needed to improve the quality and sustainability of public health care systems for all Canadians.
In support of its mandate, Health Canada conducted research, analysis and policy work on the following priorities, detailed further below: working with PHAC and federal partners to lead Canada's response to COVID-19; integrating virtual care and mental health; health expenditures and funding; primary, home and palliative care; access to sexual and reproductive health services; accessibility and affordability of pharmaceuticals; health human resources and the impacts of health care systems modernization on the health workforce; quality of care; digital health and health data; health care systems and service delivery innovation; as well as health technology.
What's New?
Health Canada's Rapid Science Snapshot Survey titled "Are the Deputy's Science Priorities on your radar?" successfully developed a baseline for progress on key elements of the Department's Framework for Science and Research Excellence. Launched in late 2021 under the leadership of the Departmental Science Advisor, this employee survey received over 1800 responses across all Branches. The data and analysis will inform future measures on science-related topics and interests across Health Canada and contribute to improving the quality, relevance and impact of science for all Canadians.
Leading Canada's response to COVID-19
Health Canada worked collaboratively with PHAC, federal departments, P/Ts, and stakeholders to lead Canada's response to the ongoing COVID-19 pandemic. The COVID Task Force played a key policy coordination role, keeping an overarching view on the many different lines of work happening across the Health Portfolio and the wider federal government. In order to keep Ministers fully-informed on public health measures and the impacts on health care system capacity under the pandemic, Health Canada's COVID Task Force continued to refine and deliver analytical products that enabled key decision-makers to quickly identify and target specific areas for action in 2021-22. This included reporting weekly on P/T public health measures and their impacts, as well as on COVID-19 developments internationally.
Health Canada led the federal, provincial and territorial (F/P/T) strategy and engagement; facilitated information sharing across jurisdictions; sought expert advice and guidance to support federal officials responsible for managing the pandemic; and led or supported multiple COVID-related intergovernmental and external advisory committees (including the Variants of Concern Leadership Group and the Federal Pandemic Science Coordination and Action Group).
In order to develop and implement innovative approaches to sharing data on human and virus genomes, the Department worked with the Canadian COVID-19 Genomics Network (CanCOGeN), supported by $40 million in funding from Innovation, Science and Economic Development Canada (ISED). The collaboration yielded the development of 2 data portals (VirusSeq & HostSeq) and the sequencing of over 400,000 viral sequences and over 7,500 host sequences as of March 2022. This pan-Canadian initiative is expected to serve as a model for sharing of knowledge, discoveries, and best practices. PHAC's National Microbiology Laboratory and CanCOGeN are implementing a transition plan to sustain this work.
With funding through CIHR ($18 million), Health Canada also helped to create the Coronavirus Variants Rapid Response Network (CoVaRR-Net) to study and communicate new information on variants of concern. CoVaRR-Net is currently laying the foundations for a broader Pandemic Preparedness Network that would facilitate collaboration between academics, public health laboratories, industry, and government decision-makers.
Health Canada worked collaboratively with federal partners to support P/Ts in protecting vulnerable populations and those at higher risk, such as temporary foreign workers living in congregate settings on farms. The Department also collaborated with Public Safety Canada and Public Services and Procurement Canada to deploy two federal Mobile Health Units in Ontario in response to a provincial request for assistance. Each unit provided 100 additional hospital beds and facilitated the transfer of non-critical care patients out of the ICU to ensure those specialized resources were available for those who needed them most.
In 2020-21, the GOC invested an unprecedented amount – roughly $7 billion – on PPE and other medical supplies. Because of the critical need for these supplies in Canada's health sector coupled with uncertainties in the supply chain, Health Canada took steps to ensure a continuous flow of deliveries, including development of an evidence-based supply-demand model, so that decision-makers could accurately tailor their procurement efforts. The GOC also supported the entry of a number of manufacturers into the marketplace and encouraged a wide range of Canadian firms to pivot, during the crisis, into manufacturing PPE. As a result of these efforts early on, Canadian supply chains stabilized over the course of 2021-22, allowing hospitals to return to procuring their own materiel and Health Canada to refocus its efforts on other areas with a greater need for coordination and support.
The Department worked closely with Public Safety Canada to establish the Humanitarian Workforce program as an essential surge support tool for P/Ts, including the sourcing of health human resources (HHR) to support hospital capacity and COVID-19 vaccination programs. In partnership with PHAC, Public Safety Canada and other federal bodies, Health Canada also worked with P/Ts and the Canadian Red Cross to provide surge capacity for such competencies as contact tracing, nursing, and outbreak management, including in northern, remote, and isolated communities. In addition, the Department maintained a roster of federal nurses available to bolster hospital capacity.
Integrating virtual care and mental health
Health Canada continued to work in collaboration with P/Ts to integrate virtual care into health services and leverage digital health tools and quality data to improve the health system and outcomes for Canadians.
The GOC provided P/Ts with $150 million over 2020-21 and 2021-22 to help accelerate efforts to meet health care needs through virtual tools and approaches, including secure messaging, video-conferencing technology and remote patient monitoring tools. In addition, Canada Health Infoway was provided $50 million to help P/Ts implement their virtual care projects, and advance pan-Canadian initiatives on virtual care standards and procurement.
In response to demands over the course of the pandemic for innovative solutions to health system challenges, digital health in Canada rapidly evolved into a fully mature industry. For example, during the first wave of the pandemic, the use of virtual care rose to 77% of outpatient visits in Ontario.
Additionally, in 2021-22, Health Canada supported a Mental Health Research Canada (MHRC) study to better understand the impacts of COVID-19 on the mental health of Canadians. MHRC collected primary data on the mental health status and needs of Canadians and the availability of supports and services throughout the pandemic. For example, data collected in February 2022 indicated that self-rated levels of high anxiety and high depression since COVID (23% and 16% of respondents respectively) continued to remain much higher than pre-COVID levels (5% and 4%, respectively).
Health expenditures and funding
Health Canada continued to research, monitor and analyze domestic and international health expenditures and funding, and their implications for health care delivery in Canada. The work informed the GOC's response to the COVID-19 pandemic, as well its overall healthcare financing strategy, by applying comparative research and analysis of the sustainability and responsiveness of Canadian and international health care delivery. The Department also developed and shared its understanding of emerging trends across health care sectors (such as long-term care [LTC] and mental health) and how to ensure the future sustainability and responsiveness of Canada's own health care system.
Primary, home and palliative care
In 2021-22, Health Canada continued to advance the GOC's commitment to take action to help people stay in their homes longer by engaging with experts and key stakeholders to develop new knowledge and facilitate the adoption of proven approaches and best practices. The Department invested in projects targeted to formal and informal caregivers and health care professionals to build capacity in primary care and equip family caregivers with the skills and resources needed to care for elderly loved ones at home. Examples include:
- Pallium's Project ECHO (Extension for Community Healthcare Outcomes): The "Increasing Primary Care Competency in Palliative Care Across Canada" project connects local health care providers with expertise and knowledge to build palliative care skills and improve access to needed palliative care services.
- Canadian Home Care Association (CHCA) for the Partners in Care: the "Preparing Family Caregivers in Supporting Patients of Home Restorative Care" program provides home care professionals with the skills to empower family caregivers with increased knowledge and confidence in delivering home-based care to frail seniors transitioning from hospital or respite to the home environment.
- McMaster University's "Building Community Paramedicine" project supports the transformation of the health system by expanding the paramedic's role in the delivery of care. Through this project, paramedics receive specialized training to conduct weekly one-on-one sessions with vulnerable older adults to improve quality of life and reduce unnecessary trips to emergency departments.
- Safe Long-Term Care Fund: The federal government invested $1 billion through the Safe Long-term Care Fund, to help P/Ts protect people from COVID-19 in long-term care settings and improve infection prevention and control. P/Ts used this funding to undertake a range of activities, including carrying out infection prevention and control readiness assessments, making improvements to ventilation and hiring additional staff or topping up wages.
Access to sexual and reproductive health services
Indigenous Peoples, racialized people, Two-Spirit, lesbian, gay, bisexual, transgender, queer, intersex, and additional sexually and gender diverse (2SLGBTQI+) people, those living with disabilities, women, and youth face the highest sexual and reproductive health risks and the greatest barriers to accessing support, information, and services. In 2021-22, Health Canada funded projects totalling approximately $15.3 million aimed at closing gaps in access to sexual and reproductive health care for these often-marginalized populations.
Currently-funded organizations (including Action Canada for Sexual Health and Rights, Egale, and the Sex Information & Education Council of Canada) are establishing baseline data to develop measurement indicators which will yield qualitative and quantitative data for fiscal year 2022-23.
Accessibility and affordability of pharmaceuticals
In 2021-22, Health Canada continued working with partners to advance initiatives supporting the affordability and accessibility of pharmaceuticals. It established the Canadian Drug Agency Transition Office which engaged relevant partners in health via nearly 200 bilateral and roundtable meetings, and developed a proposal to formally establish the Agency.
Work also continued towards developing a national strategy for drugs for rare diseases. Based on stakeholder input, the Department developed a draft framework in 2021-22 for the national strategy. Additional input is being sought via 11 additional stakeholder roundtables and continued discussions with all jurisdictions and partners in health, with a goal of launching the official strategy before the end of 2022-23.
Health human resources and the impacts of health care systems modernization on the health workforce
The pressures of the COVID-19 pandemic have brought into focus the significant challenges faced by health care workers in Canada, including increasing staffing shortages. The Department undertook both policy research and stakeholder engagement to better understand the impacts of the COVID-19 pandemic on Canada's health workforce, as well as the key challenges for the health workforce.
The Department advanced work with P/Ts and key stakeholders to: address challenges in retaining the current workforce; increase the supply of workers through recruitment, training and recognition of credentials; and understand the workforce in Canada to inform planning and decisions to support new, innovative models of care that will allow workers to maximise their skillset and leverage technology to make their jobs easier. These challenges include difficulty in retaining the current workforce, the need to build recruitment approaches for new health care workers, and build capacity to support data-driven planning. Recognizing the growing crisis in the health workforce, in 2021-22 Health Canada also launched the process to reinstate the position of a federal Chief Nursing Officer to ensure these critical health professionals were given a strong voice to help shape solutions and to increase their input in decisions affecting our health care system. Understanding that the challenges that face the health workforce cannot be met through federal action alone, Health Canada continued to serve as secretariat for the Federal/Provincial/Territorial (F/P/T) Committee on Health Workforce, a pan-Canadian forum for collaborative action that provided policy and strategic advice to jurisdictions and to the F/P/T Conference of Deputy Ministers of Health regarding HHR challenges and emerging issues.
What's New?
Funded by Health Canada, the Community-Based Research Centre's Community Health Leadership for 2SLGBTQQIA+ Sexual and Reproductive Health project created tools and resources to assist members of these communities to manage their sexual and reproductive health and foster community leadership. The Centre also created training resources to support healthcare providers in delivering safe, appropriate, high-quality sexual and reproductive health care to members of 2SLGBTQQIA+ communities. The Department also provided funding to Action Canada for Sexual Health and Rights and the National Abortion Federation Canada for projects to support access to abortion.
The Department also supported a study by the Canadian Academy of Health Sciences on the current national state of HHR that looked at the trends, challenges, and opportunities to build pan-Canadian capacity to recruit and retain staff over the next 2 to 5 years. Additionally, it funded a Foundation for Advancing Family Medicine initiative that supported efforts to expedite the integration of internationally-trained family physicians into the Canadian health care system.
Quality of Care
Health Canada worked to improve quality of care across Canada through policy research and analysis and its support for Healthcare Excellence Canada (HEC, previously the Canadian Foundation for Healthcare ImprovementFootnote 2). A new 5-year contribution agreement signed with HEC will help advance the adoption of quality and safety innovations and implementation of relevant policy change.
The Department also analysed key national issues affecting the quality of health care systems, including the timelines and associated HHR requirements applicable to clearing surgical backlogs accumulated during the pandemic. To clear the ongoing backlog in surgeries and other health procedures and support hundreds of thousands of additional surgeries, the GOC committed $2 billion in supplemental health care funding to P/Ts in March 2022. At the same time, Health Canada is engaged with P/Ts, key stakeholders and regulators on concrete ways to tackle the growing health workforce crisis. It also conducted research on timely and affordable access to services and products by analysing health care surveys and hospital administrative data.
Digital Health and Health Data
The pandemic highlighted challenges in access to timely, quality health data, critical to effective public health responses. Governments also had to adapt quickly to virtual care becoming the standard rather than the exception. In response, F/P/T jurisdictions collaborated to strengthen their digital health systems and improve health outcomes for Canadians. In 2021-22, Health Canada:
- Led a pan-Canadian conversation around building the foundation for a stable digital health ecosystem, through regular intergovernmental and expert meetings and strategic support for high-impact initiatives. As a result, F/P/T partners accelerated the use of virtual care and identified ways to work together to help Canadians receive the care they need when, where, and how they need it.
- Established a F/P/T working group to develop a national Health Data Strategy that provides a long-term vision for responding to health data and public health challenges, including: modernizing data collection and sharing; streamlining and updating the approach to privacy and access for the digital age; and clarifying accountability and governance with regard to sharing health data.
Health care systems and service delivery innovation
Recognizing the potential for health innovation to improve health care delivery and outcomes, Health Canada continued to collaborate with ISED and other stakeholders (such as Innovative Solutions Canada, the Strategic Innovation Fund, and the Digital Technology Supercluster) to support digital health solutions for individuals and improve Canadian health care systems.
For example, through Innovative Solutions Canada, Health Canada began funding 4 Canadian companies to develop and test innovations such as the use of artificial intelligence to more accurately match organ donors with recipients.
Health technology
During 2021-22, the Women's College Hospital's "National Strategy for Digital Health Evaluation" project worked towards a national strategy for digital health evaluation, which will support P/Ts in conducting evaluations of new digital health investments.
Over the course of the fiscal year, Health Canada continued to invest significantly in several pan-Canadian organizations that directly contribute to health care system improvements, including Canada Health Infoway; the Canadian Institute for Health Information; the Canadian Agency for Drugs and Technologies in Health; Healthcare Excellence Canada; and the Canada Brain Research Fund.
What's New?
Health Canada's funding for Brain Canada has supported longer-term research projects that could prove ground-breaking in the diagnosis and treatment of particular brain health conditions. For example, a $2 million research award enabled major advancements in the early detection and treatment of Autism Spectrum Disorder among at-risk infants. Another project laid the foundation in developing the world's first blood test to detect Alzheimer's disease, as well as MRI brain scan techniques to help doctors determine a prognosis. This $1.4 million project could lead to major advancements in the diagnosis of Alzheimer's.
Highlights included:
- $91.3 million to Canada Health Infoway to advance digital health innovation, including the continued development of a national e-prescribing system, virtual care initiatives, and the advancement of the organ donation and transplantation data and reporting system. Part of the funding was used to advance pan-Canadian virtual care initiatives in support of P/Ts. In addition, funding supported the adoption, use and linking of electronic medical records and their systems, improving access for providers, institutions and patients.
- $101.4 million to the Canadian Institute for Health Information (CIHI) to improve delivery of comparable and actionable data analysis and information that accelerates improvements in health care, health system performance and population health across the continuum of care. CIHI further advanced the collection of pan-Canadian data in key areas, including: home care; mental health and addictions; pharmaceuticals; as well as organ donation and transplantation. It continued efforts to support the F/P/T COVID-19 response, adjusting some of its functions to bolster access to timely, critical health system capacity data during the pandemic. Given the rapid expansion of virtual care, CIHI also launched a new web page providing information on the availability of virtual care services in Canada.
- $29.1 million to the Canadian Agency for Drugs and Technologies in Health (CADTH) to continue strengthening the management of drugs and non-drug technologies. This funding supported work aimed at improving health system effectiveness and sustainability by promoting the evidence-based, cost-effective and optimal use of drugs and other health technologies by health care decision-makers such as public drug plans and healthcare practitioners.
- $28.4 million to Healthcare Excellence Canada towards accelerating the identification, spread and scale-up of health care innovations and to improve patient safety and quality care. During 2021-22, the organization expanded its "Long Term Care Plus (LTC+) Program", reaching over 1,500 LTC facilities. By rapidly sharing information and creating a peer-to-peer learning environment, LTC+ assisted these facilities in improving safety, as well as pandemic readiness and recovery. Participants reported notable improvements in the following aspects of pandemic response: infection prevention and control practices; communication and stakeholder engagement; residents' and families' experience of care; support for staff; mental health supports; as well as planning and preparedness.
- $11.6 million to the Brain Canada Foundation (Brain Canada) and matched by the organization's private and charitable sector donors and partners. These cumulative funds supported a range of projects, several of which were completed in 2021-22 despite COVID-19 disruptions. Brain Canada's work supports discoveries with the potential to improve health outcomes for people in diverse age groups, including children and seniors. For example, one project tested and evaluated the implementation of a clinical pathway for the acute care of pediatric concussion. The research team produced tools to help physicians and nurses provide better care and improve health outcomes for children.
- $27 million to the Territorial Health Investment FundFootnote 3 to help offset medical transportation costs incurred by territorial governments, as well as to continue supporting the development and implementation of innovative activities intended to transform territorial health systems. Budget 2021 renewed the THIF for 2 years, with $54 million in total new funding for 2021-22 and 2022-23. Delays in signing grant agreements due in part due to pandemic pressures slowed the progress of some projects. Initiatives launched in 2021-22 that will continue into 2022-23 included:
- $7.1 million to the Northwest Territories, supporting: work under its Primary Health Care Reform Initiative (including demonstration projects); as well as undertaking the first of 2 years of work to implement a System Sustainability Plan, with a goal of strengthening health care service delivery in the territory;
- $6.4 million to the Yukon, supporting: development of new cultural safety and humility training; development of integrated and collaborative health service delivery models; expansion and integration of the electronic health record system; as well as implementation of transformative recommendations set out in Putting People First (the report of the Independent Expert Panel on the comprehensive review of health and social services);
- $13.5 million to Nunavut, supporting: work in priority areas of health human resources and capacity building (such as strengthening and supporting participation of Inuit paraprofessionals within the health workforce); tuberculosis program development; as well as continued implementation of the oral health program.
Did you know?
Health Canada provided funding to Healthcare Excellence Canada for its LTC+ initiative, which disseminates promising practices in preventing and mitigating the impact of COVID-19 in LTC and retirement homes. This initiative expanded to support more than 1,500 facilities across Canada in 2021-22.
Departmental Result 2: Canadians have access to appropriate and effective health services
Through Health Canada, the federal government is responsible for promoting and defending the core principles of the Canada Health Act (CHA) – public administration, comprehensiveness, universality, portability and accessibility – and ensuring provincial and territorial health care insurance plans provide reasonable access to health services without financial or other barriers, such as patient charges for insured services.
The GOC continued financial contributions to P/Ts to support publicly funded health care services through the Canada Health Transfer (more than $43.1 billion for 2021-22) and through targeted funding ($11 billion allocated in Budget 2017 over ten years) to support improved access to home and community care, and mental health and substance use services.
As part of its pandemic response, the government committed to provide an additional $3 billion to P/Ts in 2021-22 to address pandemic-related health care system pressures, particularly the backlogs of surgeries, medical procedures and diagnostics and to help jurisdictions protect people from COVID-19 in long-term care (LTC) settings and improve infection prevention and control. The GOC also directly funded an additional $20 billion in health-related pandemic response measures (e.g., for vaccines, PPE, testing and other public health support). For the period 2019-20 to 2027, the GOC has committed more than $72 billion in health related pandemic response measures.
Furthermore, with the rapid shift to virtual care and digital health services during the pandemic, Health Canada played a leadership role in facilitating pan-Canadian collaboration on strategies to improve equitable access to these services, particularly in the primary care space. For instance, in June 2021, the Department organized a summit with over 80 stakeholders and P/Ts to discuss the policy enablers underpinning delivery of virtual care services, resulting in a national action plan to maintain the unprecedented momentum spurred by the pandemic in this area.
To ensure that Canadians have access to appropriate and effective health services, including enhancing health care capacity during the pandemic, Health Canada focused its 2021-22 efforts on the following priorities, detailed further below: expanding access to mental health and substance use services; improving access to quality home, community, long-term and palliative care; supporting primary and virtual care and creating a world-class health data system; supporting testing capacity and developing innovative approaches to testing, screening and research; working towards national universal pharmacare; supporting implementation of medical assistance in dying (MAID); combatting cancer; supporting organ, tissue and blood donation and transplantation; supporting access to health services for specific populations (including Canadian thalidomide survivors support and addressing anti-Indigenous racism and discrimination in Canada's health systems).
Expanding access to mental health and substance use services
Budget 2021 provided $45 million over two years to develop an integrated suite of standards for mental health and substance use services, in collaboration with P/Ts, health organizations and key stakeholders. Health Canada signed an agreement with Standards Council of Canada to develop these standards in priority areas that align with the Common Statement of Principles on Shared Health Priorities: integration of mental health and substance use in primary care; digital mental health and substance use apps; integrated youth services; substance use treatment facilities; substance use workforce; and mental health and substance use services for complex health needs. A diverse range of key stakeholders will participate in the development process, including P/Ts, Indigenous partners, health organizations, and people with lived and living experience.
In April 2020, the Department launched the Wellness Together Canada portal to provide Canadians with free access to live and confidential online mental health and substance use resources, available 24 hours a day, 7 days a week in more than 200 languages and dialects, and with tailored support for youth, adults and frontline healthcare workers. Through the portal, individuals have immediate access to a range of supports that include assessments, self-guided programming, peer support, and counselling. In addition, a dedicated phone line allows callers to reach Program Navigators who can assist in accessing portal resources appropriate to individual needs.
Did you know?
Initially intended as a temporary urgent response to pandemic concerns, Health Canada's Wellness Together Canada portal has become a key feature of the country's mental health and substance use care landscape. As of March 2022, over 2.3 million individuals from all corners of Canada had accessed the portal in over 6.6 million web sessions. In January 2022, the portal launched the PocketWell companion app, which supports daily tracking of mood, wellbeing and mental health functionality. The new app also provides a direct link back to the portal for access to the full spectrum of mental health and substance use supports. PocketWell was downloaded 24,157 times.
Budget 2022 reflected the GOC's ongoing commitment to supporting the mental health and substance use needs of Canadians. It provided $140 million over two years, starting in 2022-23, to sustain the Wellness Together Canada portal. Wellness Together Canada has helped alleviate local pressures on service delivery by providing an alternative to in-person care. A March 2022 survey of those who accessed the portal revealed that their top three challenges are anxiety (79.4%), stress (74.5%) and depression (63.3%). Without the portal, 40% of respondents indicated that they would have considered reaching out to a general practitioner, 7% to a walk-in clinic and 5% to a hospital emergency department.
Budget 2022 also reaffirmed the government's promise to engage with P/Ts to inform the development of a new Canada Mental Health Transfer. The transfer would support the expansion and delivery of high quality and accessible mental health services across Canada, including for prevention and treatment.
The Department also directed $14.25 million to the Mental Health Commission of Canada (MHCC) to advance specific priorities in the area of mental health, substance use integration, at-risk populations, and suicide prevention. The Commission engaged with a diverse set of stakeholders – including those with lived and living experience – to develop, promote, implement, and scale mental health standards for both post-secondary students and the workplace, as well as suicide prevention programs within various jurisdictions. The organization continued to provide mental health education and training for targeted populations on addressing stigma and e-mental health.
Improving access to quality home, community, long-term and palliative care
In 2021-22, the GOC continued to fund home, community, long-term and palliative care across the country in support of the following initiatives:
- Coordinating and integrating care;
- Improving digital connectivity and the use of remote technology to access care from home;
- Providing caregivers with more education supports and expanded respite services;
- Working with all partners in health towards setting new national standards for LTC.
Did you know?
In 2021-22, the GOC reached agreements with all P/Ts for the Safe Long-term Care Fund, announced in the 2020 Fall Economic Statement, to help protect people living and working in LTC facilities and retirement homes by strengthening infection prevention and control. Federal funds contributed to such initiatives as installing and replacing air purification units, and reinforcing support for health care aid workers. For example, the funding provided an additional $2 per hour for these workers to help increase overall staffing levels in LTC facilities in Alberta.
Specifically, the GOC committed an additional $3 billion over 5 years (2022-23 to 2026-27) to support P/Ts in applying standards for LTC and making permanent changes. The funding will contribute to workforce stability and strengthen inspection and enforcement capacity and quality – and improve safety – to achieve these standards. This new funding supplements the existing $1 billion Safe Long-term Care Fund, which is helping P/Ts protect residents and staff and support infection prevention and control measures in LTC facilities and retirement homes.
These detail how each jurisdiction is using federal funding to improve infection prevention and control in key priorities areas, such as:
- Retention measures for existing staff, including wage top-ups and hiring additional HHR;
- New infrastructure and renovations to existing infrastructure, such as ventilation of self-isolation rooms and single rooms;
- Readiness assessments conducted in LTC settings to prevent COVID infections and spread.
The GOC also invested $29.8 million over 6 years for Health Canada to advance the Palliative Care Action Plan, aimed at improving access to quality palliative care, and extended the initiative to 2027. In 2021-22, the Department worked with partners to develop project proposals, initiate the foundational work for a multi-year public awareness campaign, and lay the groundwork for Indigenous-led engagement towards a distinctions-based Framework on palliative care.
Did you know?
As part of the $3 billion the GOC committed over 5 years to support P/Ts in applying standards for LTC, Health Canada funded the Health Standards Organization and the Canadian Standards Association Group to enhance stakeholder and public engagement with regard to developing national LTC standards. Once finalized, these standards will reflect the diverse perspectives of Canadians and be based on evidence-informed practices for safe, reliable, high-quality LTC.
Supporting primary and virtual care and creating a world-class health data system
Health Canada explored innovative approaches with its partners in health – including new service delivery models, and digital and virtual care solutions – to help ensure that every Canadian has access to a primary care provider or team.
The fall 2021 Mandate Letter to the Minister of Health included directives to support P/Ts in hiring new family doctors, nurses and nurse practitioners; and to expand the number of family doctors/primary health teams in rural communities. The Department undertook public opinion research in winter 2022 to assess public priorities and perceptions related to a range of primary care, team-based care, virtual care, and digital health topics, including access. Preliminary results suggest the GOC has been accurately aligning its priorities with the views of Canadians on these issues. They also highlight the importance of increasing health data literacy and opportunities for public input to build trust in health data sharing. Once finalized, the results will be leveraged in support of P/T efforts in these areas.
In spring 2021, Health Canada funded the Centre for Digital Health Evaluation (CDHE) to support its work in improving the health care experience for Canadians. In partnership with CIHI, Canada Health Infoway, CADTH, and the Centre for WISE Practices in Indigenous Health, CDHE is: establishing a new digital health evaluation network that includes academics, patient groups, decision-makers, and other stakeholders; and creating a digital health evaluation framework with standardized methods.
Also during 2021-22, the Department worked with P/Ts to implement the Diagnostic Services Policy as part of the compliance with the provision of the CHA, which ensures that patients are not charged fees for diagnostic services. The policy came into effect in April 2020. Due to the retroactive nature of the CHA reporting, P/Ts will begin reporting on compliance with the Policy in December 2022.
Making virtual care a concrete reality
For 2021-22, Canada Health Infoway received $50 million in funding to support P/Ts in implementing and expanding virtual care initiatives in their jurisdictions. This funding enabled the organization to collaborate with F/P/T administrations on strategic investments, interoperability enablement, change management, and procurement support in order to accelerate deployment or scaling of digital health and virtual care solutions. Infoway also worked towards developing pan-Canadian standards for secure messaging and video-conferencing, and advanced pan-Canadian initiatives in the area of standards, procurement, and change management.
P/Ts have made tremendous strides in virtual care services by implementing tools and supports into their health systems. Focused on accelerating this work, the GOC announced a $240.5 million investment in May 2020 to support implementing, maintaining and expanding virtual care and mental health tools to facilitate Canadians accessing necessary care during COVID-19 and beyond. By March 2022, $150 million of this funding had flowed directly to P/Ts via bilateral agreements, in continued support of their efforts in the following shared priority areas:
- Secure messaging and information-sharing;
- Secure video-conferencing technology;
- Remote patient monitoring tools;
- Patient access to COVID-19 and other lab results;
- Integration and alignment of new platforms, tools and approaches into existing digital health systems.
This agenda was driven by historic F/P/T collaboration via a dedicated Virtual Care and Digital Table with a mandate to consider and develop a national plan to accelerate deployment of virtual care, both during the pandemic and beyond. Through this Table and its working groups, Health Canada sought guidance and feedback from relevant F/P/Ts and external experts. These efforts helped strengthen the funding agreements and their alignment with federal priorities, including digital government, meeting the needs of priority populations, transparency, and demonstrating results.
Supporting testing capacity and developing innovative approaches to testing, screening and research
Health Canada continued to invest in the Safe Restart Agreement (SRA) in the areas of testing and data management improvements to address COVID-19 directly and to create sustainable systems able to respond to future health emergencies. In 2021-22, the Department committed $156.8 million and spent $155.1 million towards 33 projects in the following areas: developing distribution channels for rapid antigen tests and conducting clinical trials for their use; testing and wastewater surveillance research and infrastructure; as well as strengthening data management for P/Ts and Indigenous organizations.
Did you know?
Through the SRA Contribution Program, Health Canada supported investments in clinical trials for Canadian testing data to approve rapid antigen tests, and created distribution channels and workplace screening program supports for small and medium enterprises, large organizations, private industry and vulnerable populations. Through an MOU with Statistics Canada, the Department continued to provide contact tracing surge support for P/Ts, at points of entry and for Indigenous communities.
Specifically, Health Canada provided $5 million for 12 wastewater surveillance projects across 4 P/Ts, $146 million to modernize and improve the interoperability of health and public health data collection and management across 10 P/Ts, and $22.4 million to 5 national Indigenous organizations to modernize and improve the interoperability of Indigenous health and public health data collection and management.
The Department also supported the Greater Toronto Airports Authority and partner universities to pilot an innovative wastewater surveillance program to detect COVID-19 variants of concern in aircraft and terminal sewage water, and invested $2.7 million in 5 testing clinical trials and studies to support Canadian data collection for medical device regulatory approvals and off-label use of tests during the pandemic.
The Department drew from external science and policy experts to develop and refine existing and innovative approaches to testing, including through the COVID-19 Testing and Screening Expert Advisory Panel; Industry Advisory Roundtable on COVID-19 Testing, Screening, Tracing and Data Management; and the Testing and Screening Knowledge Exchange.
Federal Direct Distribution of Rapid Tests
Health Canada met its objective of significantly increasing screening with rapid tests in workplaces across Canada as a way to reduce transmission and outbreaks of COVID-19 in concert with other public health measures. The Department procured and distributed over 402 million rapid tests to P/Ts so that Canada had the testing capacity needed to support reopening the economy.
In early 2021, Health Canada implemented distribution channels to supply rapid testing kits to the private sector for workplace screening, in coordination with P/T initiatives. The increase in workplace testing provided an essential added layer of protection to Canadians, as did ensuring that tests were made accessible to vulnerable individuals. As a result, approximately 12.6 million tests were provided for workplace screening, among which:
- Over 6.6 million tests were distributed to some 950 enterprises with 200 employees and more;
- Over 5.2 million tests were distributed to pharmacies in 6 provinces for access by more than 13,000 enterprises with under 200 employees;
- Over 700,000 tests were provided to the Canadian Red Cross for access by more than 1,000 charities and non-profit organizations.
Health Canada also worked with partners to help ensure tests were accessible to vulnerable individuals across the country. As a result:
- Approximately 3.2 million tests were provided to the Canadian Red Cross for distribution to individuals by more than 640 participating organizations;
- Residents of 39 Northern communities were able to access more than 300,000 tests in their local grocery stores;
- More than 1.7 million rapid tests were distributed to First Nations and Northern, Remote and Isolated (NRI) communities through Indigenous Services Canada (ISC), in collaboration with the National Microbiology Laboratory;
- The Department provided rapid tests to airports and railways that connect either to or from remote communities in order to support Transport Canada's policy on proof of vaccination for remote communities.
Health Canada also facilitated the roll-out of mandatory testing in support of the COVID-19 vaccination requirement for federal public servants to approximately 100 departments and agencies, and facilitated the expansion of voluntary testing to over one-third of these organizations.
Working towards national universal pharmacare
In 2021-22, Health Canada's Canadian Drug Agency Transition Office invested $1.3 million of the $35 million which was committed over 4 years in Budget 2019, towards personnel and operating costs (including a contract for reviewing the pharmaceuticals data landscape). Transition Office staff conducted significant engagement via nearly 200 bilateral and roundtable meetings with relevant F/P/Ts, partner organizations, Indigenous organizations, patient advocates, experts, and international leaders in pharmaceuticals management. They then analysed the qualitative and quantitative evidence and developed a proposal to formally establish the Agency.
To support commitments to develop a national strategy for drugs for rare diseases, the Department produced a draft framework, based on stakeholder input, and held a series of 10 stakeholder roundtables. Discussions and collaboration continued with all relevant partners in health towards finalizing the strategy, expected to launch in 2022-23.
Health Canada also provided funding to the Canadian Agency for Drugs and Technologies in Health (CADTH) to continue strengthening the management of drugs and non-drug technologies. To support ongoing work on the foundational elements of national pharmacare, CADTH created an advisory panel to propose a framework for developing a potential pan-Canadian prescription drug list, or formulary. Following public consultations this winter, the panel's final report and recommendations were submitted to the Department and publicly released in June 2022.
To inform the advancement of national universal pharmacare, the GOC signed an agreement with the Government of Prince Edward Island (PEI) to provide $35 million over 4 years to help add new drugs to its list of covered drugs, and lower out of pocket costs for drugs covered under existing public plans for Island residents. Lessons from PEI's efforts to improve accessibility and affordability of pharmaceuticals will help inform ongoing work to advance national universal pharmacare.
Supporting implementation of medical assistance in dying (MAID)
In order to meet federal commitments to support the new MAID legislation passed in March 2021, Health Canada collaborated with P/Ts towards its implementation. In July 2021, using information collected under the federal MAID monitoring program, the Department released the "Second Annual Report on Medical Assistance in Dying (MAID) in Canada, 2020". This report provided Canadians with information about the circumstances under which people are requesting MAID and insight into its application across the country.
Health Canada provided funding to the Canadian Association of MAID Assessors and Providers to support the development of training and guidance materials for practitioners to facilitate consistent and safe access. As well, it worked with the Department of Justice to prepare and consult on a set of revised federal regulations for the MAID monitoring program. The Department also supported an Expert Panel on MAID and Mental Illness, as required by legislation, to report to Ministers of Health and Justice.
Combatting Cancer
In 2021-22, Health Canada provided over $4.7 million to The Terry Fox Research Institute to grow its national network of cancer centres with expertise in advancing precision medicine in cancer. The data generated continues to form an important resource for Canadian cancer research.
What's New?
Health Canada funding supported the Canadian Partnership Against Cancer's collaboration with the international modelling community to develop and apply a modeling tool to estimate the impact of cancer care service interruptions and delays during the COVID-19 pandemic on future cancer cases in Canada. The Partnership analyzed and compared various catch-up strategies toward optimizing health care system cancer screening and reducing the impacts of pandemic-related service interruptions.
The Department provided $2.25 million to Ovarian Cancer Canada towards addressing gaps in knowledge about ovarian cancer. Funding was used to develop experimental models, test new treatments, and conduct various research projects, all contributing to increased knowledge of effective treatment options for ovarian cancer, including advancements in precision medicine as a management tool.
Health Canada also invested $52.5 million in the Canadian Partnership Against Cancer to continue working collaboratively with its partners – P/Ts, cancer programs and agencies, health organizations, Indigenous agencies and other key stakeholders – to leverage collective efforts in support of equitable access to quality, sustainable cancer care.
Supporting organ, tissue and blood donation and transplantation
The Department worked with stakeholders through the Organ Donation and Transplantation Collaborative to support priorities that advance transformative, system-level improvements. 2021-22 outcomes included:
- Developing a preliminary foundation of a governance model (including a defined role for patients) with various stakeholders, and P/Ts and appropriate representation of the Quebec Ministry of Health (Ministère de la santé et des services sociaux) and Transplant Quebec;
- Conducting preliminary analysis on the impact of deemed consent in Nova Scotia, which shaped implementation and communication strategies for the law and system reforms in the province;
- Systemically mapping out Canadian research in organ donation and transplantation to create a more effective system;
- Developing new knowledge on the integration of patient-reported outcome measures and on barriers to accessing kidney transplantation among populations marginalized by race and ethnicity.
Health Canada provided $3.58 million to Canadian Blood Services to produce new knowledge that increases understanding of the family experience with deceased organ donation in Canada and contributes to addressing burnout in donor coordinators and supporting their resilience. Funding also supported:
- A public education strategy to promote the national education and learning resource portal for teachers, students and parents, launched in 2021;
- A knowledge exchange event to promote a shared understanding of the diversity, equity and inclusion initiatives underway in the organ, tissue and blood donation and transplantation system;
- The COVID-19 Impact Dashboard, which informed the Clinical Practice Guideline for Solid Organ Donation and Transplantation During the COVID-19 Pandemic.
In addition, with Health Canada funding, the Canadian Institute for Health Information and Canada Health Infoway continued to work towards modernizing the organ donation and transplantation data and performance reporting system, to improve decision-making and fill current data and information gaps.
Supporting access to health services for specific populations
In 2021-2022, the Department invested $38.8 million under the Official Languages Health Program to community-based organizations, governments and academic institutions to improve access to health services for English-speaking communities in Quebec and French-speaking communities elsewhere in Canada.
In addition, the Program supported a number of innovative projects across Canada that took into account the specific needs of patients in official language minority communities (OLMCs), including home care and mental health. These projects seek to better understand the needs of these communities and improve their access to health services in the official language of their choice. Key results included:
- Association des collèges et universités de la francophonie canadienne (ACUFC)-Consortium national de formation en santé (CNFS) and McGill University increased the number of bilingual health providers delivering services in OLMCs. The ACUFC-CNFS recorded 838 additional health graduates from 104 distinct health programs. McGill University enrolled over 1,750 health and social services professionals in its Dialogue McGill's English-language training courses, 1,477 of whom completed their courses, for a success rate of 85%.
- The Société Santé en français (SSF) supported francophone minority communities (outside Quebec), and the Community Health and Social Services Networks supported English-speaking minority communities (in Quebec) by undertaking networking initiatives that mobilized partners in health to improve access to services within OLMCs. A total of 39 community-based health networks and 10 satellites across Canada collaborated with various health sector stakeholders to improve community access to health services in their official language of choice.
- Health Canada also supported innovative projects to improve access to health services for OLMCs in relation to such F/P/T health priorities as mental health and home care.
Canadian thalidomide survivors support
The Department continued its contribution to meeting the lifetime needs of Canadian thalidomide survivors, allowing them to age with dignity. The Canadian Thalidomide Survivors Support Program maintained its 3-step probability-based medical assessment process to determine eligibility. By March 2022, 238 applications had been submitted and of those, 41 were new. Overall, the Program provided support to 123 thalidomide survivors in 2021-22. The application period remains open until June 2024.
Based on needs identified in the 2020-21 annual survey of survivors and gaps identified by the administrator, the program introduced a new paperless process for survivors requesting annual reassessment of their disability level. Survivors could schedule a call with a healthcare professional to discuss their physical health needs and challenges, offering a more personalised approach. In 2021-22, a total of 32 survivors requested reassessment using this process, compared to less than 4 in previous years under the paper-based approach.
Addressing Anti-Indigenous Racism and Discrimination in Canada's Health Systems
In 2021-22, Health Canada undertook further action to foster health systems free from racism and discrimination, where Indigenous Peoples are respected and safe, by implementing the priorities of the Federal Pathway to Address Missing and Murdered Indigenous Women, Girls and 2SLGBTQQIA+ People and the United Nations Declaration on the Rights of Indigenous Peoples. These efforts included:
- Supporting the 3rd National Dialogues to Address Anti-Indigenous Racism in Canada's Health Systems;
- Developing a new Addressing Racism and Discrimination in Canada's Health Systems Program, which began flowing $13.9 million over 3 years for systems-level, community-supported projects aimed at combating anti-Indigenous racism in Canada's health systems.
Key risk for Core Responsibility 1: Health Care Systems
Information on Key Risks is available on Health Canada's website.
Results for Core Responsibility 1: Health Care Systems
The following tables show, for Core Responsibility 1: Health Care Systems, the results achieved, the performance indicators, the targets and the target dates for 2021–22, and the actual results for the three most recent fiscal years for which these results are available.
| Departmental result indicators | Target | Date to achieve target | Actual results |
|---|---|---|---|
| National health expenditure as a percentage of Gross Domestic Product (GDP) (Baseline: 10.9% of GDP in 2014-15) |
Between 10.4% and 12.8% | March 31, 2022 |
|
| Real per capita health expenditureTable dr1 Footnote 1 (Baseline: $4,049 per person in 2014-15) |
Between $3,913 and $4,782 | March 31, 2022 |
|
| Drug spending as a percentage of Gross Domestic ProductTable dr1 Footnote 2 (Baseline: 1.7% in 2014-15) |
Between 1% and 2% | March 31, 2022 |
|
| Percentage of family physicians using electronic medical records (Baseline: 73% in 2015) |
At least 95% | March 31, 2022 |
|
|
|||
| Departmental result indicators | Target | Date to achieve target | Actual results |
|---|---|---|---|
| Percentage of Canadians (aged 15+) with a mental disorder who have expressed that they have an unmet mental health care need (Baseline: 26% in 2012) |
At most 22% | March 31, 2022 |
|
| Percentage of Canadians (aged 18+) who have expressed that they have an unmet need for access to home care services (Baseline: 1.6% in 2015-16) |
At most 1% | March 31, 2027 |
|
| Percentage of Canada Health Act compliance issues addressed within 24 months of identification (Baseline: 53% in 2016-17) |
At least 95% | March 31, 2022 |
|
| Percentage of Canadians (aged 12+) who did not fill a prescription for medicine or skipped doses of medicine because of the cost (Baseline: 7.1% in 2014) |
At most 5% | March 31, 2022 |
|
|
|||
Budgetary financial resources (dollars) for Core Responsibility 1: Health Care Systems
The following table shows, for Core Responsibility 1: Health Care Systems, budgetary spending for 2021–22, as well as actual spending for that year.
| 2021–22 main estimates |
2021–22 planned spending |
2021–22 total authorities available for use |
2021–22 actual spending (authorities used) |
2021–22 difference (actual spending minus planned spending) |
|---|---|---|---|---|
| 2,456,807,897 | 2,456,807,897 | 7,906,983,369 | 4,744,300,568 | 2,287,492,671 |
Note: The variance of $2.3 billion between actual and planned spending is mainly due to the following: Additional in-year funding of $5.5 billion mainly to continue Health Canada's response related to the COVID-19 pandemic for: the procurement of additional COVID-19 rapid test kits; investments in LTC; supporting emergency measures related to the pandemic; the Safe Restart Agreement for federal investments in testing, contact tracing and data management; improving mental health supports and services; as well as drugs, medical devices and virtual care. This is offset by $3.2 billion mainly resulting from the repurpose and reprofile of funds to support emergency measures related to the pandemic; as well as Health Canada requesting both statutory and voted spending authorities allowing for flexibility for the procurement of critical and time-sensitive additional COVID-19 rapid tests. These expenses were charged to either the statutory or the voted authorities – not both. For any expenses charged to the statutory authorities, an equal amount was frozen in the voted appropriations. |
||||
Human resources (full-time equivalents) for Core Responsibility 1: Health Care Systems
The following table shows, in full-time equivalents, the human resources the Department needed to fulfill this core responsibility for 2021–22.
| 2021–22 planned full-time equivalents |
2021–22 actual full-time equivalents |
2021–22 difference (actual full-time equivalents minus planned full-time equivalents) |
|---|---|---|
| 276 | 428 | 152 |
Note: The variance in full time equivalent utilization is mainly due to Health Canada's response to the COVID-19 pandemic. |
||
Financial, human resources and performance information for Health Canada's Program Inventory is available in GC InfoBase.
Core Responsibility 2: Health Protection and Promotion
Description
Health Canada works with domestic and international partners to assess, manage and communicate the health and safety risks and benefits associated with health and consumer products, food, chemicals, pesticides, environmental factors, tobacco and vaping products, cannabis, and controlled substances.
The United Nations 2030 Agenda for Sustainable Development and UN Sustainable Development Goals (SDGs)
Health Canada's results under Core Responsibility 2: Health Protection and Promotion directly supported Canada's efforts to address the UN 2030 Agenda and the related Sustainable Development Goals (SDGs).
The Department provided information to Canadians on healthy eating, prioritizing vulnerable populations at risk of food insecurity, and collaborated internationally on food safety and nutrition standards, supporting (SDG 2) and (SDG 3). For example:
- It raised awareness about healthy eating among children and youth, particularly populations vulnerable to food insecurity. A collaboration with the University of Guelph brought together colleges and universities across Canada to create principles and criteria that encourage greater access to healthier food on campuses, with a particular focus on students who are food insecure. This supports CIF Ambitions "Canadians have access to sufficient, affordable and nutritious food" (SDG 2) and "Canadians Adopt Healthy Behaviours" (SDG 3) and the related CIF indicator 3.1.1.
Health Canada also ensured access to safe, effective and quality health products by introducing innovative and agile regulatory measures, and supported healthier living and protection from unsafe substances by funding initiatives across Canada to promote the good health and well-being of Canadians (SDG 3). For example:
- It established measures to expedite the regulatory review of COVID-19 health products without compromising safety, efficacy and quality standards; monitored the supply and demand of certain drugs and medical devices; and introduced regulations to help mitigate and prevent shortages, where possible. This supported the CIF Ambition "Canada prevents causes of premature death".
- It supported initiatives across Canada to help prevent, reduce or treat the harms associated with the use of a range of controlled drugs and substances including opioids, stimulants, cannabis, alcohol, tobacco, and vaping products, reaching communities at greatest risk or who may face barriers accessing services. This supports the CIF Ambitions "Canadians adopt healthy behaviours" and "Canada prevents causes of premature death" and related CIF indicators 3.2.1, 3.4.1, 3.12.1, and 3.13.1.
The Department published Canadian drinking water guidelines to improve drinking water quality (SDG 6). All jurisdictions in Canada consult and use these regularly-updated guidelines and guidance documents to establish their drinking water requirements. This supported the CIF Ambition "Canadians have access to drinking water and use it in a sustainable manner".
In addition, Health Canada conducted research to examine whether exposure to ambient air pollution affects the incidence and severity of COVID-19, to support the goal of making cities and human settlements inclusive, safe, resilient and sustainable (SDG 11). For example:
- New research showed associations between short-term exposure to ambient air pollutants and COVID-19 emergency department visits. It was also found that exposure to air pollution may lead to more severe COVID-19 disease. This supported the CIF Ambition "Canadians live in healthy, accessible, and sustainable cities and communities" and related CIF indicator 11.3.1.
The Department continued to work with partner departments to facilitate access to safe and sustainable use of pesticide products, through the application of current science, contributing to Canada's efforts towards sustainable consumption and production patterns (SDG 12). This supported the CIF Ambition "Canadians consume in a sustainable manner".
Furthermore, Health Canada continued to build capacity through initiatives with P/Ts to support the health sector in preparing for and adapting to the impacts of climate change (SDG 13). For example:
- It provided project funding to 10 health authorities across Canada via HealthADAPT (including the First Nations Health Authority to assess climate change impacts on Indigenous marine food safety and vulnerability of BC Coastal First Nations to develop local and Indigenous-specific adaptation strategies). This supported the CIF Ambition "Canadians are well-equipped and resilient to face the effects of climate change."
Results
The Department continued to advance the Regulatory Innovation Agenda in 2021-22, a multi-year regulatory modernization plan designed to make the federal regulatory framework more agile and responsive to an innovative environment, while ensuring the system remains science and safety-based.
The Agenda cuts across multiple Departmental Results that make up Core Responsibility 2. Its implementation will result in health product and food regulatory frameworks that protect the health and safety of Canadians with oversight proportional to any associated risks, while encouraging innovation.
Health Canada remained committed to implementing its Health and Biosciences Roadmap. Temporary regulatory measures were launched to help health and bioscience companies bring urgently-needed medical devices and vaccines for COVID-19 to market that provided an opportunity to pilot more agile regulatory solutions, such as a broader use of terms and conditions. The Department is building on this experience and lessons learned to inform policy and regulatory development as it continues to implement Roadmap commitments. Other Roadmap achievements in 2021-22 included:
- Consulting and publishing "What We Heard Reports" on a proposal to modernize the regulatory framework for clinical trials related to human drugs, medical devices and natural health products; and on a proposed regulatory framework for clinical trials involving foods for a special dietary purpose;
- Advancing the policy and operational work towards implementing a new regulatory framework for advanced therapeutic products;
- Publishing a Notice of Intent in the Canada Gazette, Part I to inform and engage stakeholders on proposed amendments to the Food and Drug Regulations in support of regulatory agility;
- Expanding Mutual Recognition Agreements with the European Union, recognizing certification from more partners, and supporting the implementation of the Canada-U.K. Trade continuity agreement;
- Strengthening the Pest Management Regulatory Agency's human and environmental health and safety oversight of pesticides and protection of human health and the environment, including wildlife, through a number of key activities organized around: improved transparency; increased use of real-world data and independent advice; modernized business processes for pesticide reviews; and a targeted review of the Pest Control Products Act;
- Publishing final regulations allowing the sale of human milk fortifiers in the Canada Gazette, Part II;
- Pre-publishing a draft regulatory framework for supplemented foods in the Canada Gazette, Part I to protect the health and safety of Canadians while providing a predictable regulatory environment for industry;
- Publishing amendments to the Food and Drug Regulations and Natural Health Products Regulations in Canada Gazette, Part II to improve alignment with foreign jurisdictions and reduce burden for industry by reducing the records retention requirement for sponsors of clinical trials from 25 years to 15 years.
Departmental Result 3: Canadians have access to safe, effective and quality health products
In 2021-22, Health Canada continued to make significant investments towards ensuring Canadians had timely access to safe, effective and quality health products – including prescription and non-prescription pharmaceutical drugs, biologic and radiopharmaceutical drugs, natural health products, and medical devices – and to meet the needs of the health care system. These investments will help accelerate market access for innovative, breakthrough products along with cost-effective alternatives, such as generic and biosimilar drugs.
The Department focused on the following priorities, detailed further below: providing ongoing access to COVID-related health products; promoting timely access to other health products; managing and monitoring drug and medical device shortages; modernizing the way we provide access to drugs not readily available; applying real-world evidence (RWE) to support regulatory decision-making; strengthening regulatory oversight; modernizing compliance and enforcement; acting to prevent and control antimicrobial resistance (AMR); fostering international collaboration and coordination; as well as promoting access to new and emerging technologies.
Providing ongoing access to COVID-related health products
Health Canada continued to work closely with domestic and international partners to meet the needs of Canadians for COVID-related health products. The Department provided regulatory advice for clinical trials, medical device reviews and drug reviews. This included guidance on the management of COVID-19 clinical trials during the pandemic, expedited review of clinical trial applications, and advice on the regulatory requirements for COVID-related medical devices, drugs and vaccines.
What's New?
Health Canada launched a new Drug and Health Product Portal in February 2022 that provides consumers with detailed information about authorized health products. The Department also began developing a similar mobile application. The first mobile release, planned for 2022-23, will focus on COVID-19 vaccines and treatments. Both tools will help empower consumers to make informed decisions on health products.
Domestic Biomanufacturing
Health Canada provided expertise on Good Manufacturing Practices (GMP) to support the GOC priority to increase domestic biomanufacturing capacity, including specialized advice and guidance to meet the unique needs of pharmaceutical companies developing or entering the Canadian biomanufacturing market.
Clinical Trials
Health Canada maintained the flexibilities and efficiencies for clinical trials related to COVID-19 drugs and medical devices initially implemented in early 2020. In May 2021, the Minister of Health made a second Interim Order extending the authorizations, obligations and oversight for another year and, in March 2022, the Clinical Trials for Medical Devices and Drugs relating to COVID-19 Regulations were published, making the changes permanent. The Department also updated the accompanying guidance document to support applications for COVID-19 drug clinical trials under the new Regulations.
To support rapid assessment of promising COVID-19 therapies, Health Canada prioritized and expedited the reviews of clinical trial applications for these products (14 days instead of the standard 30), without compromising on health and safety. As of March 2022, the Department had authorized 116 clinical trials for potential COVID-19 treatments and vaccines and 37 for medical devices.
Did you know?
As part of its commitment to openness and transparency, Health Canada continued to publish high-level summaries of the evidence that it reviewed prior to authorizing a product. The Department also expedited the release of clinical information for COVID-19 vaccines and treatments, often in partnership with the EU and other partners. Publicly releasing this type of information supports Canada's objective for transparent decision-making, enables re-evaluation of the data and fosters new research questions that could help with the use or development of drugs and medical devices.
Vaccines and other drugs
In 2021-22, Health Canada authorized new COVID-19 vaccines, and changes to them, including for Comirnaty, Spikevax, Janssen COVID-19 Vaccine, Covifenz, Nuvaxovid and Vaxzevria. The Department also authorized new to COVID-19 therapies, and changes to them, including for Sotrovimab, Evusheld, casirivimab / imdevimab and Paxlovid, which was approved in January 2022 as the first oral treatment for COVID-19. In addition, the Department continued to expedite the issuing of drug establishment licences to ensure supply chains involved in the manufacturing, importation and distribution of drugs and other critical medicines continued to supply the Canadian market. It issued more than 3,600 drug establishment licences, 112 of which were directly related to COVID-19 or medically-necessary drugs.
Health Canada continued to work closely with PHAC to monitor the safety and effectiveness of COVID-19 vaccines and other products and to rapidly investigate and mitigate any risks. For example, the Department proactively conducted environmental scans, reviewed monthly summary safety reports, requested additional safety information from manufacturers, monitored and assessed adverse reaction reports, detected any emerging safety issues, and requested information on the effectiveness of products against emerging variants.
When necessary, Health Canada imposed new terms and conditions; updated product safety information; revised risk management plans; issued risk communications; and updated product labelling to increase awareness among the public and health professionals. For example, the Department:
- Reviewed Risk Management Plans for COVID-19 vaccines and treatments, namely Paxlovid, Covifenz and Nuvaxovid and assessed the risks associated with expanding access of the Moderna and Pfizer vaccines to children;
- Closely monitored, reviewed and assessed reports of adverse events related to COVID-19 vaccines and other health products, including the off-label use of products used to treat COVID-19;
- Published the document "Guidance for market authorization requirements for COVID-19 drugs" to support manufacturers seeking market authorization for these products in understanding the requirements to demonstrate safety, efficacy and quality;
- Disseminated 18 COVID-19 "Health Product Risk Communications" and 30 COVID-19 "Health Product InfoWatch" articles targeted to healthcare professionals.
Medical Devices
In response to the ongoing high demand in 2021-22 for medical devices like diagnostic tests, PPE and ventilators, Health Canada continued to support the expedited review and authorization of COVID-19 medical devices through Interim Orders, including the most recent in March 2022. These actions ensured ongoing access to safe and effective COVID-related medical devices for Canadians.
In 2021-22, the Department received 125 applications for COVID-related Medical Device Establishment Licenses, from which it issued 88. In addition, it conducted 288 remote compliance assessments of Medical Device Establishments, including 257 domestic and 31 foreign establishments.
During the same period, Health Canada received 4,218 applications for COVID-related medical devices and authorized 793. Of these authorizations, 400 were for PPE, 114 for test kits, 67 for thermometers, 22 for ventilators, and 190 for other devices. The Department also authorized 53 COVID testing devices, including 11 self-tests along with 3 saliva collection devices and kits.
In response to the unprecedented demand for certain supplies, including medical masks and respirators, Health Canada collaborated with Employment and Social Development Canada and the Canadian Standards Association to update regulations (first on a temporary basis, then permanently) regarding the use of respirator masks, based on a new made-in-Canada standard, and published updated information for health professionals.
Did you know?
In 2021-22 Health Canada protected the health of Canadians by countering misinformation regarding ivermectin as a drug to prevent or treat COVID-19. The human version of ivermectin is authorized for sale in Canada only for the treatment of parasitic worm infections in people; there is no evidence that it works for COVID-19 and the drug is not authorized for this use. The veterinary version of ivermectin, especially at high doses, can be dangerous for humans and may cause serious health problems and even death.
The Department issued several public advisories to serve as a source of trusted information, including an urgent update following increasing reports from Canadian poison centres regarding the improper use of ivermectin. A summary of relevant advertising incidents was published online to support openness and transparency of Health Canada's regulatory actions.
Sanitizers, Disinfectant and Cleaning Products
Increased demand for sanitizers, disinfectants and cleaning products as a result of the pandemic continued through 2021-22. Health Canada maintained interim measures and guidance to increase the availability of, and access to, certain workplace and household cleaning products and soaps, which in turn helped Canadians continue to combat the virus.
The Department reviewed 27 new hard and soft surface sanitizers and 49 sanitizing devices, and authorized 1,190 new disinfectants. The interim measure for importation and sale of biocides was extended to December 2022 to continue supporting Canadian industry. As a result, the online list of hard-surface disinfectants effective against COVID-19 now contains over 700 products. Regulatory flexibilities for the importation and sale of hand sanitizers (eventually discontinued once product availability stabilized) and hard surface disinfectants resulted in the exceptional importation of approximately 400 products.
Health Canada also assessed hand sanitizers containing 0.3% benzalkonium chloride and recalled those that may have posed health risks. Safety alerts were posted on the Department's website to alert consumers of these potential risks. From the onset of the pandemic to the end of March 2022, Health Canada authorized over 4,400 new hand sanitizer products, 875 COVID-related disinfectants, and 2,088 new site licences.
Promoting timely access to other health products
In addition to facilitating access to COVID-related health products in 2021-22, the Department continued to provide Canadians with timely access to other health products by reviewing the safety, efficacy and quality of pharmaceutical and biologic drugs, medical devices and natural health products.
Health Canada approved 70 new prescription pharmaceutical drugs for human use, of which 22 contained new active substances not previously approved in Canada, as well as 6 new veterinary drugs for use in companion and food-producing animals. It also approved 176 new generic drugs for human use and 11 new veterinary generic drugs. As well, the Department approved 31 new biologic drugs for human use, of which 13 were drugs containing new active substances not previously approved in Canada. Furthermore, market authorizations were issued for 10 biosimilar drugs (ones demonstrated to be highly similar to a biologic drug previously authorized in Canada).
Health Canada also approved 556 requests for significant changes (referred to as 'supplements') to pharmaceutical drugs for human use already on the market (not including generic drugs) and 281 supplements for significant changes to biologic drugs for human use already on the market. These supplements included changes such as new uses, new safety updates to the labelling material, new manufacturing methods, and new dosing recommendations for existing drugs.
In addition, the Department approved a total of 19,951 natural health products, including hand sanitizers, 1,376 new Class II, III, or IV medical devices, as well as 396 requests for significant changes to medical devices already on the market, such as design changes and new manufacturing methods.
Did you know?
Certain drugs may face logistical or ethical challenges in terms of conducting human clinical trials. However, Health Canada can use special regulatory mechanisms in extraordinary situations. In November 2021, Health Canada authorized TPOXX (Tecovirimat), the first treatment approved in Canada for smallpox. TPOXX did not undergo human clinical trials, as it was a key ethical concern in that it would require participants be exposed to the variola virus, which causes smallpox. Instead, Health Canada used data from animal models to determine the drug's efficacy. Since smallpox is also a potential bioterrorism weapon, this authorization also served as an emergency preparedness measure.
In January 2022, Health Canada published a notice to clarify requirements for researchers who wish to conduct clinical trials with psilocybin. This naturally occurring psychoactive drug is being studied by researchers in Canada and internationally for its potential to treat various conditions, such as anxiety, depression, obsessive-compulsive disorder and substance use disorder. The notice clarifies that clinical trial approval may be granted in situations where the psilocybin is produced under appropriate conditions, adhering to GMP, and there is evidence of safe use for the intended purpose.
In order to improve the dissemination of health product safety information, allowing Canadians to make better informed decisions regarding their health, the Department updated the current email notification system, introducing a new platform with improved functions and technology.
Health Canada consulted with stakeholders to continue advancing changes to the Food and Drug Regulations while collaborating with ISED to advance related amendments to the Patented Medicines (Notice of Compliance) Regulations that would support greater availability of lower-cost generic drugs for Canadians and improve alignment with comparable international regulators.
Did you know?
In 2021-22, Health Canada initiated activities related to the Pediatric Drug Action Plan, such as advancing development of policy towards a future regulation to require pediatric-specific studies when a drug is expected to be used for children. This would bring Health Canada into closer alignment with other trusted international regulators, including the U.S. and the E.U.
The Department continued to align its drug submission review processes in 2021-22 with those of Health Technology Assessment (HTA) organizations, as part of the Regulatory Review of Drugs and Devices initiative. As of March 2022, over 80 reviews had been aligned between CADTH, l'Institut national d'excellence en santé et en services sociaux, and Health Canada. Such alignment often leads to shorter intervals between Health Canada authorizations and HTA reimbursement recommendations related to drug coverage, ultimately contributing to faster access to new drugs for Canadians.
In addition, the Department continued to conduct GMP inspections of facilities involved in the manufacturing of health products, including 316 domestic inspections and 15 foreign onsite inspections.
Managing and monitoring drug and medical device shortages
In partnership with P/Ts, industry, and patient/health care groups, Health Canada played an ongoing leadership role and took action in 2021-22 on addressing critical national drug and medical device shortages to ensure Canadians had access to the medicines and devices they needed.
The Department remade, modified, or made permanent most of the temporary regulatory measures (Interim Orders) implemented during the pandemic. This allowed it to continue using exceptional importation and sale of therapeutic products that are manufactured to comparable standards in trusted foreign jurisdictions to secure additional supplies for Canadians.
Health Canada continued to respond to pandemic-related shortages. New regulatory provisions came into effect to help safeguard the Canadian drug and medical devices supply and allow the Department to: require specific drug shortage-related information from a company and mandatory reporting of medical device shortages; permanently establish measures to source foreign supplies in critical shortage; and prevent foreign importation frameworks from causing or worsening a drug shortage in Canada.
Specific 2021-22 initiatives with regard to drugs included:
- Improving drug shortage information-sharing with stakeholders, including co-chairing biweekly national discussions and the Multi-Stakeholder Steering Committee on Drug Shortages;
- Working collaboratively with external stakeholders to permanently implement the Tier Assessment Committee, which designated 19 drugs as Tier 3 critical, national shortages;
- Adding 11 drugs to Health Canada's list of designated drugs to facilitate importation under Interim Orders, and monitoring the supply and demand of over 500 drugs deemed critical to treat hospitalized COVID patients;
- Chairing an international drug shortages working group with other regulatory bodies to share shortage information, mitigation strategies, and opportunities to align and coordinate;
- Establishing a time-limited (May 2020-June 2022) COVID-19 Critical Drug Reserve with P/Ts to mitigate shortages of 12 critical COVID-19 treatments. This Reserve served as an additional tool in the country's collective pandemic response efforts.
Did you know?
In 2021-22, the Critical Drug Reserve backstopped P/T inventories by increasing the Canadian supply of drugs needed to support patients with COVID-19 that were vulnerable to shortages. P/Ts used the Reserve as "shortage insurance" against demand surges through waves 4, 5, and 6 of the pandemic, and as a mechanism to cooperatively reallocate drugs between jurisdictions to where they were needed most to avoid localized shortages. Over the fiscal year, the Reserve inventory helped avoid or mitigate shortages for 5 of its 12 listed drugs.
Specific 2021-22 initiatives with regard to medical devices included:
- Operationalizing amendments to the Medical Devices Regulations requiring manufacturers and importers to report shortages and discontinuations that may lead to shortages of medical devices that are on Health Canada's List of Medical Devices – Notification of Shortages. As of March 2022, 406 shortage reports and 1 discontinuation report had been posted to the list. This information enabled the Department to better manage medical device shortages and support Canadians' access to these devices;
- Monitoring shortages of medical devices critical to the COVID-19 pandemic response, to help mitigate and manage shortages where possible.
Modernizing the way we provide access to drugs not readily available
Health Canada continued policy work on future regulatory amendments regarding the use of foreign decisions, towards achieving the best balance possible between access to drugs and ensuring their safety and efficacy.
The Department also worked with both PHAC and the Canadian Armed Forces to advance efforts to finalize a new regulatory framework that will enable public health officials responsible for public and military health emergencies to request access to drugs that are unavailable in Canada, for emergency preparedness and response purposes.
The Special Access Program allows health care professionals to request, in emergency situations, medical devices and drugs not yet authorized for use in Canada. In 2021-22, Health Canada developed a tool to allow these professionals to submit requests electronically, follow up on the status of requests, and receive decisions electronically via their mobile devices. External testing of the tool began in early 2022.
Applying real-world evidence (RWE) to support regulatory decision-making
RWE is evidence regarding the use, and potential benefits or risks, of a medical product that is gathered after a product is on the market. In 2021-22, the Department collaborated with domestic and international partners to advance the use of RWE. For example, Health Canada participated on the Canadian RWE Steering Committee to help develop a pan-Canadian RWE approach as well as contributed to the newly-established International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) M14 working group. The Department also worked with the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) on ways to collaborate and harmonize regulations in this area.
The Department also began to collect examples of RWE in pre-market health product submissions as a precursor to developing guidance.
In addition, Health Canada continued to collaborate with other trusted international regulators to apply RWE during the COVID-19 pandemic by co-chairing and participating in several working groups and launching 4 COVID-19 research studies. These ongoing studies and analyses (expected to be completed in 2022-23) will provide valuable insights on the impacts of COVID-19 within the Canadian population. In addition, aligning research protocols between international regulators enhances collaboration and helps to develop processes and approaches for joint analyses across jurisdictions.
Did you know?
Health Canada established new regulatory processes to authorize medications to treat substance use. For example, in 2021-22, Health Canada approved diacetylmorphine hydrochloride as a supervised injectable opioid to be used for adult patients with severe opioid use disorder. Further, new regulations allow health care practitioners to provide this treatment outside of a hospital setting, if permitted by their P/T jurisdiction.
Strengthening regulatory oversight
In 2021-22, the Department implemented new regulations related to the post-market surveillance of medical device safety and effectiveness. Supporting guidance documents were developed for: incident reporting; foreign risk notification; summary reports and issue-related analyses; and guidance related to new authorities. Health Canada also launched a GCwiki page to support industry, and made multiple presentations at the Canadian Medical Devices Industry Conference.
In June 2021, the Department published a regulatory proposal to improve the labelling of natural health products in Canada Gazette, Part I for a 90-day public comment period. It also initiated an Over-the-Counter Drug Action Plan to support efforts to modernize the regulatory framework for non-prescription ("over-the-counter") drugs in advance of regulatory amendments. This includes non-regulatory changes to policy, guidance and procedure documents.
Heath Canada consulted stakeholders about creating a modern regulatory framework for biocides with more flexibility in tailoring application and regulatory requirements specific to these products, in order to maintain a stable supply of safe and effective surface sanitizers and disinfectants in Canada. This approach would enable the Department to apply regulatory decisions by other trusted international regulators, facilitate entry of innovative products, promote trade and eliminate duplicative reviews.
In 2021-22, Health Canada continued to implement regulations that require hospitals to report all medical device incidents and serious adverse drug reactions. The Department established quarterly updates for hospitals and continued national and regional outreach activities to support hospital regulatory compliance. In 2021-22, hospitals submitted an average of 92 incident reports per month related to medical devices, and an average of 363 reports per month for adverse drug reactions. This reporting allowed the Department to continue monitoring the ongoing safety and effectiveness of these products.
Health Canada also proposed ways to: modernize Medical Device Establishment Licensing and better align recall requirements of the Medical Devices Regulations with those of other trusted international partners and regulators; and strengthen compliance and enforcement by implementing terms and conditions and partial suspensions and by refining the risk-based approach to recall reporting.
To further support compliance and enforcement efforts, the Department conducted 36 inspections coupled with extensive stakeholder consultation under the Natural Health Product GMP inspection pilot project and developed guidance documents and policies.
Health Canada continued to fund the Canadian Medication Incident Reporting and Prevention System in partnership with the Institute for Safe Medication Practices Canada, the Canadian Institute for Health Information, and Healthcare Excellence Canada. The initiative, which aims to reduce potential or actual harm caused by preventable medication incidents, saw 11 safety bulletins and 11 consumer newsletters published over the fiscal year in response to reported medication safety concerns, including those emerging during the pandemic.
Modernizing compliance and enforcement
In 2021-22, Health Canada maintained its commitment to becoming more agile, assertive, consistent, innovative, proactive, and risk-based as part of its Compliance and Enforcement (C&E) Modernization and Transformation priority, by:
- Strengthening its data and analytical capacity to better inform regulatory oversight decision-making;
- Updating the Department's designation framework to ensure that the inspectors and analysts who enforce Acts and regulations have the optimal level of expertise, training and certification;
- Updating and replacing IT systems that support compliance and enforcement activities.
Did you know?
Health Canada piloted an Artificial Intelligence platform to proactively monitor natural health products making illegal, false, or misleading claims of preventing, treating, or curing cancer. The platform was also used to proactively monitor drugs and devices making illegal, false, or misleading claims of preventing, treating, or curing COVID-19. In both situations, the Department took enforcement actions on claims not following the Canadian regulatory advertising framework.
In response to COVID-related public health restrictions and measures, Health Canada continued to adopt remote and virtual tools for some C&E activities, including compliance verification, Good Pharmacovigilance Practice inspections, and delivering health and safety risk assessments to protect Canadians. It piloted the VidCruiter platform for remote and hybrid inspections, used platforms such as MS Teams and Zoom, and transitioned all of its training for inspectors to an online environment.
Acting to prevent and control antimicrobial resistance (AMR)
Recognizing the urgent need to monitor, prevent and mitigate the serious and growing threat of AMR for the health of humans, animals and the environment, and in accordance with the Federal Action Plan on Antimicrobial Resistance and Use in Canada and the Pan-Canadian Framework for Action on Tackling Antimicrobial Resistance and Antimicrobial Use, Health Canada undertook a number of initiatives in 2021-22 to preserve the effectiveness of the antimicrobials Canadians rely upon every day while monitoring and supporting their prudent use in animals.
In response to the Minister's mandate commitment, the Department continued to collaborate with international partners to resolve AMR issues, including the International Coalition of Medicines Regulatory Authorities (ICMRA), the Transatlantic Task Forces on Antimicrobial Resistance (TATFAR) and other bilateral/multilateral partnerships. In 2021-22, Health Canada renewed its commitment to TATFAR by contributing to and adopting a revised work plan for 2021-2026; and championed the launch of the ICMRA Best Practices project which will share information on proven or promising regulatory and non-regulatory measures to address AMR.
Health Canada launched a public consultation to update its List of certain antimicrobial active pharmaceutical ingredients. This list (which had not been updated since its inception in 2017) comprises ingredients that are considered important in human medicine. The update included adding 52 ingredients and the reclassification of two existing agents.
Other key actions to combat AMR in 2021-22 included:
- Updating the Pathogens of Interest List to include fungal pathogens, an emerging threat;
- Re-evaluating, on a targeted basis, labels of medically-important antimicrobials for use in animals with unspecified or prolonged durations of use, following the post-market re-evaluation framework of veterinary drugs published in 2020;
- Publishing, in collaboration with PHAC, the 2019 and 2020 Highlights Reports summarizing data submitted through the Veterinary Antimicrobial Sales Reporting system. Health Canada now has published 3 years of antimicrobial sales data collected under mandatory requirements that are helping to inform its ongoing antimicrobial surveillance, stewardship, and responsible use initiatives;
- Facilitating access to low-risk veterinary health products to improve health and wellness in animals and to reduce the need for routine use of antimicrobials. In collaboration with the CFIA, Health Canada continued incremental progress to allow the use of low-risk veterinary health products in livestock feed, a pathway not previously available to livestock owners. With completion of the first phase of this pilot project, 12 such products were authorized to be mixed in animal feeds;
- Holding the Best Brains Exchange (BBE) in October 2021 and publishing the subsequent "Challenges in the antimicrobial business model and potential incentives to increase access and promote innovation" report from BBE in March 2022.
Fostering international collaboration and coordination
Health Canada continued to collaborate with international partners and participate on various international committees over 2021-22, driven in part by the continued need for treatments and vaccines to combat COVID-19.
Throughout the pandemic, the Department leveraged existing collaborative international relationships to share more information in real-time. It participated in international discussions on safety and quality issues observed with COVID-19 medical devices, and in the COVID-19 Vaccine Pharmacovigilance Network of the International Coalition of Medicines Regulatory Authorities, which facilitated timely access to information about COVID-19 vaccines in other jurisdictions and supported Health Canada's own assessments and actions.
Did you know?
In January 2022, Health Canada authorized Paxlovid, the first oral tablet therapy to treat mild to moderate COVID-19 in patients at high risk of progressing to serious disease, including hospitalization or death. Previously-authorized COVID-19 treatments had to be administered in a hospital or healthcare setting, but Paxlovid is a tablet that can be taken at home twice per day for 5 days. It combines 2 antiviral drugs intended for use as soon as possible after diagnosis of COVID-19 and within 5 days of the start of symptoms, and works by stopping the virus from replicating. The Department successfully expedited the review of this drug submission while coordinating with international regulatory partners, and enabled its immediate distribution upon authorization, making it available to Canadians as early as possible.
Medical Devices
Health Canada and the U.S. FDA resumed a pilot project towards developing a Medical Device Single Review Program to improve patient access to medical devices, support innovation, and strengthen the development of standards. The Department continued to participate in the International Medical Device Regulators Forum, which aims to harmonize regulatory requirements for medical devices, and concluded a 3-year term as chair of the Medical Device Single Audit Program, which seeks to improve the safety and oversight of medical devices on an international scale.
In addition, Health Canada participated in the International Medical Devices Regulators Forum Adverse Events Working Group, which aims to maintain harmonized terminology for reporting adverse events related to medical devices and define a core international dataset for collection.
Drugs (pre-market)
Health Canada continued to support access to drugs via bilateral arrangements with other trusted international regulators and multilateral initiatives/networks. Project Orbis continued to strengthen the Department's international collaborations in the review of drug submissions, risk management plans, and surveillance. The launch of the Health Canada Project Orbis Webpage provided increased transparency about the initiative.
To promote international alignment and greater efficiencies in the drug review process, the Australia-Canada-Singapore-Switzerland-U.K. Access Consortium released its Strategic Plan for 2021 – 2024 and published the Operational Procedures for New Active Substances Work-Sharing Initiative. The Consortium also expanded work-sharing to biosimilar drugs and published a statement on COVID-19 medicines outlining its commitment to only authorize those medicines whose benefits outweigh the risks and to continually monitor these products for safety, efficacy and quality.
In January 2021, Canada and the U.K. also published new guidance on the veterinary drug simultaneous review processes for regulatory cooperation. This collaboration creates opportunities for manufacturers to access two major markets simultaneously, helps to expand treatment options for animals, and supports the global competitiveness of food producers.
In 2021-22, Health Canada continued to foster international collaboration and coordination by:
- Serving as an observer of the Committee for Medicinal Products for Human use, allowing the Department to exchange and seek information as needed;
- Serving as a member of the International Council for Harmonisation Assembly and Management Committee and chairing its Finance Committee;
- Co-chairing the International Coalition of Medicines Regulatory Authorities COVID-19 working group, supporting collaboration and alignment of common policy positions and scientific requirements for clinical trials and approvals of relevant drugs and vaccines;
- Collaborating with the FDA to develop a common approach to rare diseases, incorporating the patient's perspective, and to build clinical trial readiness;
- Participating on the International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products steering committee and on the Codex Alimentarius committee responsible for setting standards for veterinary drug residues in foods;
- Chairing an international drug shortages working group that included representatives from the U.S., U.K., Europe, Australia, Japan and the World Health Organization (WHO). The group met regularly to monitor the drug supply situation and share best practices related to shortage mitigation policies and strategies.
Did you know?
Health Canada has been contributing as an advisor to the work of an international consortium (Setting International Standards of Patient-Reported Outcomes and Quality of Life Endpoints in Cancer Clinical Trials) since January 2021. The goal of this consortium is to improve how we use patient feedback about their well-being and functioning while on new cancer therapies when making regulatory decisions.
Drugs (post-market)
The Department participated in regular international fora, including the EMA's
Pharmacovigilance Risk Assessment Committee, the Pharmacovigilance Cluster Teleconference, and the International Post-Market Surveillance Teleconference, sharing information between the agencies on pharmacovigilance and post-market surveillance. Regulators discussed subjects such as signal tracking, incident reporting, and adverse effects of medical products.
Health Canada also collaborated with trusted international regulators on pharmacovigilance issues of mutual priority through more than 60 ad-hoc incoming and outgoing international information requests.
Compliance and Enforcement
The Department expanded its international partnership with the European Union as well as the scope of its Mutual Recognition Agreements (MRA) to recognize and accept certificates of compliance for foreign building inspections conducted by our MRA partners.
In addition, Health Canada shared information regarding health product recalls with its partners; participated as a member of the International Coalition of Medicines Regulatory Authorities; and was appointed Chair of the Pharmaceutical Inspection Co-Operation Scheme for the next two years.
The Department fostered international collaboration in the context of the pandemic by exchanging critical information with key partners for the oversight of the global drug supply chain.
Research
Health Canada conducts research in partnership with other federal agencies and international experts to address issues related to infectious diseases and their associated vaccines. This work has been published in peer-reviewed journals that support the advancement and regulation of vaccines against COVID-19, influenza, and respiratory syncytial virus (RSV). Topics covered in 2021-22 included novel vaccine platforms using DNA, and recombinant vector vaccines against COVID-19 and RSV.
Promoting access to new and emerging technologies
Scientific and technological advances are accelerating the pace of innovation in the health care system, leading to the development of innovative health products that use emerging technologies such as advanced artificial intelligence (AI) and machine learning (ML) algorithms, telerobotics, 3D printing and gene editing. To keep pace with emerging technologies, Health Canada has led international working groups, has been both a national and international leader in machine learning and cybersecurity, and is advancing policy in the digital health space. Specific highlights for 2021-22 included:
- Presenting at and participating in a U.K.-led G7 technical working group on AI governance; member states published two complementary papers on AI/ML-enabled medical devices;
- Co-authoring a joint statement with the U.S. FDA and the U.K.'s Medicines and Healthcare products Regulatory Agency related to Good Machine Learning Practices, to promote safe, effective and high-quality medical devices that use AI and ML.
The Department continued implementation of the new regulatory framework for Advanced Therapeutic Products (ATP), allowing it to authorize these in a more flexible and risk-based manner. In a first exploration of its use, stakeholders provided input towards applying the framework to adaptive machine learning-enabled medical devices (devices that "learn" and are intended to be frequently updated to improve performance). Health Canada then secured feedback from the Scientific Advisory Committee on Digital Health Technologies and created an External Reference Group of technical experts and stakeholders.
The Department established another External Reference Group to help tailor regulatory requirements for a second potential ATP candidate – fecal microbiota therapy for recurrent C. difficile infection.
Given the complexity involved, Health Canada recognized that innovators require additional support when seeking authorization for these novel products. The Department has committed to providing enhanced client services, known as a "Concierge Service", which consists of identifying a primary contact at Health Canada who helps guide the applicant through the review process.
Departmental Result 4: Canadians are protected from unsafe consumer and commercial products and substances
Helping Canadians lead healthier lives and providing protection from unsafe consumer and commercial products and substances remained a vital component of Health Canada's work. Over the course of 2021-22, the Department's efforts in this regard focused on the following priorities, detailed further below: applying a comprehensive approach to substance use-related harms; regulating cannabis; managing the health risks of chemicals in the home, the workplace and the environment; supporting the safety of consumer products and cosmetics; protecting Canadians from radiation; as well as strengthening pesticide regulation and transparency.
Applying a comprehensive approach to substance use-related harms
Substance use-related harms continue to cause devastating health and social effects on Canadians from every walk of life.The overdose crisis in particular continues to have significant impacts on Canadian communities and families. For example, between January 2016 and December 2021, coroners' reports recorded 29,052 lives lost to apparent opioid toxicity with a disproportionate impact on men, individuals between the ages of 20-59, and Indigenous Peoples.
Did you know?
Funding provided to P/Ts through the Emergency Treatment Fund contributed to: a significant reduction of wait times in 5 jurisdictions (Manitoba, New Brunswick, Newfoundland and Labrador, Northwest Territories, and Nova Scotia); new treatment beds in British Columbia and Ontario; On-the-Land Healing Camps in Nunavut; and expanded access to Rapid Access Addiction Medicine clinics in British Columbia, Ontario and Saskatchewan. Funding also contributed to: training additional health care workers across the country, thereby increasing the available pool of treatment providers; improving overall quality of care; building community-level capacity; expanding access to virtual supports; and addressing methamphetamine use.
While the opioid and stimulant related harms and deaths were trending downward towards the end of 2019 and beginning of 2020, several jurisdictions across Canada saw an alarming reversal in the months following the onset of COVID-19, which continued over the last two years resulting in record-breaking levels of deaths and harms. In 2021, the death rate per day increased to approximately 21 deaths per day in comparison to pre-pandemic levels that ranged from 7 (in 2016) to 12 (in 2018). Contributing factors included increased feelings of isolation, stress and anxiety, and limited availability or accessibility of services for those who used drugs during the pandemic.
People who use drugs also experienced higher risks related to an increasingly toxic illegal drug supply, with fentanyl and its analogues comprising the largest share of opioids submitted for analysis by law enforcement. Of all accidental apparent opioid toxicity deaths between January – December 2021, 86% involved fentanyl and 82% involved opioids that were only non-pharmaceutical. The pandemic contributed to the increasingly toxic drug supply due to travel restrictions and the implementation of border measures, which disrupted the supply chain. In addition to fentanyl, some P/Ts faced an increase in benzodiazepine and non-opioid sedatives contaminating the illegal drug supply. This made it more difficult to respond to overdoses as, unlike fentanyl, these drugs do not respond to naloxone. COVID-related public health restrictions further compounded risks for people who use drugs by reducing access to treatment and harm reduction services. Recognizing these impacts, Health Canada supported greater access to services offering safer, pharmaceutical-grade alternatives to the highly-toxic illegal drug supply.
In 2021-22, Health Canada worked closely with P/Ts to continue implementing the Emergency Treatment Fund, established in Budget 2018 to provide one-time emergency funding of $150 million via bilateral agreements and action plans to match P/T investments that, combined, will see more than $300 million directed towards improving access to treatment in the context of the opioid overdose crisis. By the end of 2021-22, P/Ts had invested over 87% of the funding towards supporting treatment in all its forms in their respective jurisdictions.
In 2021-22, Health Canada continued its work to provide evidence-informed policy and regulatory direction related to substance use, monitor substance use, including alcohol, support harm reduction initiatives, and modernize the policies and operational procedures for supervised consumption sites (SCS) and services. Achievements included:
- Monitoring trends in the use of alcohol and psychoactive pharmaceuticals, and illegal drugs (including cocaine or crack, ecstasy, speed or methamphetamines, hallucinogens, heroin, and others) through the 2019 Canadian Alcohol and Drugs Survey and the 2019-20 Canadian Post-Secondary Education Alcohol and Drugs Survey;
- Monitoring trends in the use of tobacco and vaping products through the Canadian Tobacco Survey and the Canadian Community Health Survey;
- Monitoring hospitalizations from opioid- and stimulant-related poisonings and reporting on findings as part of the Opioid- and Stimulant-related Harms in Canada quarterly update from the Special Advisory Committee on the Epidemic of Opioid Overdoses;
- Extending until September 2025 the class exemptions allowing all P/Ts to establish urgent public health need sites and other harm reduction measures in response to local needs;
- Extending until September 2026 the class exemption authorizing health care practitioners, including nurse practitioners, to prescribe controlled substances verbally; allowing pharmacists to prescribe, sell or provide controlled substances in limited circumstances; and allowing pharmacy employees to deliver prescriptions to patients;
- Authorizing SCS to allow drug splitting/sharing where the appropriate policies and procedures are in place;
- Authorizing SCS to allow for the transportation of samples for the purposes of drug checking (a harm reduction measure where people have their drugs tested for toxic or unexpected and potent substances like fentanyl);
- Amending regulations to allow health care practitioners to request access to restricted drugs on behalf of patients, including psilocybin and MDMA, through Health Canada's Special Access Program (SAP);
- An Expert Task Force on Substance Use was established in March 2021, with a mandate to provide Health Canada with independent advice and recommendations on the GOC's drug policy and potential alternatives to criminal penalties for the simple possession of controlled substances. The task force reports are available on the HC External Advisory Body website;
- Providing over $20 million, through Health Canada's Substance Use and Addictions Program (SUAP), to support 18 safer supply projects in British Columbia, New Brunswick, Ontario, and Quebec. These projects provide pharmaceutical-grade drugs as an alternative to the highly-toxic illegal drug supply to help prevent overdoses and save lives, as part of a continuum of care that may include other substance use treatment and harm reduction services. The Department continued to work with partners to expand the evidence-based treatment service and support the scaling up of effective models;
- Initiating the process to develop national mental health and substance use service standards to improve access to services and address issues such as safety, effectiveness, and cultural appropriateness.
In an effort to reduce barriers to treatment, Health Canada continued to invest in public health campaigns that focused on reducing the stigma of substance use. Specific investments were made to better understand and target high risk groups, such as young and middle aged men, to reduce stigma and promote help-seeking behaviour. The Department also collaborated with international partners (including the United Nations Commission on Narcotic Drugs) to develop a better understanding of stigma of and improve access to treatment services.
The Canadian Drugs and Substances Strategy outlines the GOC's approach to substance use with the goal of minimizing the harms to individuals, families and communities. It covers a broad range of substances, including alcohol, cannabis, illegal drugs, and prescription drugs.
Health Canada continued to work on updating the Strategy to reflect expert advice, lessons and feedback from public consultations on drug policy undertaken in 2018, ministerial outreach to stakeholders in winter 2022, the Ministerial Expert Task Force on Substance Use, the Canadian Pain Task Force, and other important inputs, to strengthen the GOC's approach. This will incorporate urgent responses to address the ongoing overdose crisis and impacts of the COVID-19 pandemic on substance use in Canada, such as providing regulatory approval for SCS, facilitating access to safer supply services, and strengthening overdose prevention efforts, including increased access to naloxone kits.
Did you know?
Unmanaged chronic pain is a contributing factor of substance use and a barrier to the treatment of related disorders. With Health Canada's support, the Canadian Pain Task Force published its third and final report in 2021, which provided over 150 recommendations for better prevention and management of chronic pain across Canada.
Over the fiscal year, the Department continued to promote, monitor, verify and enforce compliance with the Controlled Drugs and Substances Act. It monitored the supply chain, including licensed dealers and pharmacies, to ensure controlled substances and precursor chemicals were handled appropriately and remained in legal distribution channels.
Health Canada conducted 166 inspections of licensed dealers, following up on an additional 19 compliance verifications where potential concerns were identified. The Department conducted 89 inspections of pharmacies and followed up on an additional 217 compliance verifications to mitigate the risk of diversion of controlled substances to the illegal drug supply market. It also developed a compliance promotion strategy to expand its reach in ensuring that pharmacies comply with legal requirements.
In response to the worsening overdose crisis and a surge in opioid-related deaths, Health Canada funded 38 new projects across the country via SUAP, including those providing frontline services in the COVID-19 context.
In the first 6 months of 2021-22, SUAP-funded projects delivered a total of almost 48,000 knowledge products and learning opportunities, reaching approximately 8 million Canadians and focusing on: adults and youth (who use drugs and/or who are at-risk, peers, Indigenous Peoples, 2SLGBTQI+, racialized, alcohol-use related disorders, low-income); front-line care teams and healthcare professionals; F/P/T, regional and municipal governments; school boards/trustees; community partners; non-profit organizations; program designers; policy makers; and the general public.
Did you know?
In 2021-22, Health Canada invested over $85 million through SUAPFootnote 4 for 180 substance use projects across Canada. This funding supported a wide range of evidence-informed and innovative projects that focused on substance use prevention, harm reduction, and treatment as it relates to opioids, stimulants, cannabis, alcohol, tobacco and vaping products. These projects also delivered new knowledge products and learning opportunities that reached several million Canadians and stakeholders.
In 2021-22, Health Canada also invested over $20 million towards 18 pilot projects designed to provide pharmaceutical-grade drugs as safer alternatives to the contaminated illegal drug supply in Canada (safer supply projects) as well as develop a safer supply community of practice. SUAP also provided over $2 million towards 13 projects exploring new approaches to addressing methamphetamine use. An additional $5.8 million funded the distribution of naloxone kits and opioid overdose response training to support communities particularly hard hit by the crisis.
Through SUAP, the Department directed $11 million to the Canadian Centre on Substance Use and Addiction (CCSA) to address substance use in Canada. With this funding, CCSA advanced work in key areas contributing to federal priorities on substance use: developing and disseminating new knowledge to improve awareness, creating tools and resources, and working with partners to support the development of standards and guidelines. The Centre's estimates of the costs and harms of substance use continue to inform policy makers as to how to allocate resources in their respective jurisdictions.
Funded through SUAP, CCSA continued to make an impact on reducing alcohol-related harms by updating Canada's Low-Risk Drinking Guidelines, to better reflect the latest scientific evidence on alcohol-related risks and harms. When released in 2022-23, these renewed guidelines will provide Canadians with the most accurate and up-to-date information on lower-risk alcohol use. This work complements the Department's contributions to developing the Global Alcohol Action Plan 2022-30, approved in January 2022, to strengthen implementation of the Global Strategy to Reduce the Harmful Use of Alcohol.
Health Canada also partnered with CCSA to produce a toolkit to support Canadians working in trades and industries that are physically demanding and may reinforce heavy substance use. This toolkit provides employers and workers with educational material and access to services and support for prevention and protection from substance use.
CCSA's work on updating the Competencies for Canada's Substance Use Workforce directly supported a departmental priority related to health human resources. These tools and resources have been partially or fully adopted by numerous stakeholders and jurisdictions. CCSA also complemented Health Canada's efforts to reduce stigma by connecting on this topic with more than 40 universities and colleges, law enforcement agencies, and people and community groups with lived and living experience of substance use.
In 2021-22, Health Canada continued supporting public health organizations by analysing drug samples. The Department also continued to notify Canadian law enforcement agencies and public health partners about newly-identified drugs in these analyses and increased its collaborations with various international partners to harmonize the way drugs are classified and how the data is reported.
Health Canada continued to strengthen its engagement with Canadians who have experienced substance use issues through its People with Lived and Living Experience Council. The Council continued to provide advice to deepen understanding of this community and help inform the Department's policies and programs. For example, the Council advised the Department on funding priorities and evaluation criteria in the 2021 SUAP call for proposals and reviewed these proposals from the perspective of their community.
Health Canada hosted its Public Education Partnership Symposium on Substance Use in May 2021. This event brought together public education professionals in a virtual setting, including individuals and organizations who represented those with lived and living experience. The Symposium focused on the intersection between the COVID-19 pandemic and substance use.
The Department led a joint GOC and law enforcement working group that discussed the precursors used in the illegal manufacturing of methamphetamine to help inform options to address precursors of concern identified by law enforcement. Health Canada also participated in a North American Drug Dialogue Science initiative promoting collaboration between Canada, the U.S. and Mexico regarding methamphetamine, fentanyl and other precursors. A white paper that outlines innovative or rapid surveillance approaches taken to track and understand impacts on substance use and overdose during the COVID-19 pandemic was also produced as part of the 2021 Roadmap for a Renewed Canada-U.S. Partnership. These collaborations ensured that all jurisdictions were fully up-to-date and presented opportunities for coordinated action.
Regulating cannabis
The Department continued to support the effective implementation and objectives of the Cannabis Act to protect public health and public safety – in particular, the health of young persons by restricting their access to cannabis – while providing adults with legal access to regulated products and reducing illegal activities involving cannabis.
To meet these objectives, Health Canada worked in close collaboration with other federal departments, P/Ts, Indigenous communities, the regulated industry, public health organizations, academics, international partners, and law enforcement. It also initiated planning for the mandated review of the administration and operation of the Act.
What's New?
Health Canada continued to implement strategies aimed at promoting a diverse commercial cannabis industry. Through its dedicated Indigenous Navigator service, the Department issued in 2021-22 an additional 20 licenses for cultivating or processing cannabis to Indigenous-owned or affiliated applicants located across Canada, for a total of 48 licensed Indigenous businesses. It also awarded an additional 14 licences to Indigenous-owned or affiliated applicants to cultivate or process industrial hemp, for a total of 33.
In support of restricting youth access, Health Canada closely monitored usage rates of cannabis. Evidence across multiple Canadian surveys (e.g., Canadian Cannabis Survey, Canadian Student Tobacco Alcohol and Drugs Survey, Canadian Community Health Survey, and the Canadian Alcohol and Drugs Survey) suggests that the prevalence of cannabis use among youth has remained stable. The Department also continued to monitor compliance with regulations that reduce the appeal to youth, including plain packaging and labelling.
A tightly-regulated cannabis industry capable of delivering a sufficient supply of quality-controlled products continued to be in place in 2021-22 and Health Canada observed progress towards the displacement of the illegal cannabis market. Federally-regulated production of cannabis continued to expand and diversify. The Department granted an additional 256 licences for cultivation, processing, and/or sale of cannabis for medical purposes, 151 licences for research, analytical testing, and/or cannabis drug, and 157 for industrial hemp. It continued to track the movement of cannabis throughout the supply chain and published regular market data on its website to keep Canadians informed of industry trends.
Health Canada continued to facilitate reasonable access to cannabis for medical purposes by registering individuals who have the support of their health care practitioner to produce a limited amount of cannabis for their own medical purposes.
At the same time, the Department strengthened oversight of the program to reduce the risk of abuse on the average daily amount of cannabis for medical purposes authorized by health care practitioners for personal and designated production. For example, Health Canada began actively seeking additional evidence from health care practitioners to substantiate high authorizations, and using its authority to refuse a registration where there is a risk to public health and public safety. The Department has increased compliance monitoring and support to law enforcement, and is sharing data and meeting with P/T regulatory authorities to discuss concerning trends with high authorizations. Data on cannabis for medical purposes is available online.
Health Canada continued to promote, monitor, verify and enforce compliance with legislative and regulatory requirements. The Department is responsible for regulatory oversight of the legal industry while law enforcement agencies are responsible for enforcing the criminal prohibitions associated with cannabis.
What's New?
Health Canada consulted and sought feedback from Canadians through numerous cannabis public consultations, including the proposed Regulations Amending the Cannabis Regulations (Flavours in Cannabis Extracts), the proposed Regulations Amending Certain Regulations Concerning Cannabis Research and Testing and Cannabis Beverages, and Guidance on Personal Production of Cannabis for Medical Purposes, which set out proposed factors Health Canada may consider in refusing or revoking a registration on public health and public safety grounds. A What We Heard report and the final guidance was published in April 2022.
In 2021-22, Health Canada undertook over 2,200 compliance promotion activities (e.g., emails, webinars, calls, letters), reviewed a total of 25,587 notices of new cannabis products and followed up on 7% of them due to potential non-compliance with the regulations, investigated over 1,400 cannabis complaints, and made 211 referrals to law enforcement for possible enforcement action.
The Department also conducted 5 compliance monitoring projects to review industry compliance in areas such as vaping and edible cannabis products. It conducted 410 inspections with an overall industry compliance rate of 98%, completed 173 compliance verifications, undertook 317 cannabis compliance promotion sessions, and collected 319 cannabis product samples for laboratory analysis to monitor and enforce compliance with legislative and regulatory requirements.
In 2021-22, Health Canada visited 56% of current cannabis license holders for either an inspection, compliance verification, sampling activities, compliance promotion, or a combination of these activities.
The number of inspection actions greatly increased over the previous year (2020-21) and targeted a wider range of industry activities, including compliance monitoring projects related to cannabis vaping, cannabis extracts, and edible cannabis products. This expansion in number and scope allowed for greater industry oversight to ensure Canadians continued to have safe access to a quality-controlled and increasingly diverse range of cannabis products.
Managing the health risks of chemicals in the home, the workplace and the environment
As part of its commitment to implementing the Chemicals Management Plan (CMP), Health Canada continued to assess chemical substances and manage identified risks to help protect Canadians and the environment, including sub-populations who may experience greater susceptibility or exposure to harmful chemicals.
Through the CMP, the GOC assesses and manages risks posed by chemical substances that can be found in food and food products, consumer products, cosmetics, drugs, drinking water and industrial releases. For example, Health Canada and the CFIA conduct regular surveillance of the levels of certain contaminants in the Canadian food supply. The Department also establishes maximum levels for contaminants to minimize dietary exposure to food contaminants.
Did you know?
To increase the efficiency of the Workplace Hazardous Products Program and improve outcomes for workers and claimants alike, Health Canada continued to modernize its approach to the review of confidential business information claims under the Hazardous Materials Information Review Act. This included updating the application and payment processes, streamlining the review process, allowing electronic delivery of documents, and updating the publication process.
In keeping with the Minister's mandate letter, Health Canada continued to partner with Environment and Climate Change Canada to strengthen the Canadian Environmental Protection Act (CEPA), 1999. Proposed amendments to the Act were included in Bill C-28 in April 2021 and in Bill S-5 in February 2022. The Department also proposed amendments to the Food and Drugs Act, in Bill C-28 and Bill S-5, to strengthen the environmental risk assessment and risk management of drugs. These amendments are part of Health Canada's approach to modernizing the Food and Drug Regulations with regard to the environmental risk assessment of active ingredients in drugs. The 2 departments continued their collaboration to assess all new substances (373 in 2021-22) before these are imported into or manufactured in Canada under the authority of the Act. Where either department identified risks, measures to manage these were instituted. The pair also continued to assess the safety of existing substances under the CMP, with approximately 94% (4,139 substances) of the program's total planned assessments completed by the end of March 2022.
Health Canada also continued to respond to recommendations made in the Workplace Hazardous Products Program Evaluation, including improving communication and guidance materials as well as exploring potential resources to better measure and monitor the Program's impact. For example, during inspections, the Department provided industry with a new information package including links and resources to information on the Workplace Hazardous Materials Information System and technical guidance and educational materials on the Hazardous Products Act and its Regulations.
Internationally, Health Canada collaborated with global partners in 2021-22 to advance the adoption, implementation and updating of the Globally Harmonized System of Classification and Labelling of Chemicals to facilitate international trade while promoting the safe use of chemical products. As well, the Department was designated a WHO Collaborating Centre on Environmental Health for 4 years (2021-25), which facilitates international collaboration on managing the health effects of chemical exposures as well as other environmental risk factors such as air pollution, drinking water quality and climate change.
In December 2021, Health Canada released the Sixth Report on Human Biomonitoring of Environmental Chemicals in Canada. The Report provides human biomonitoring results from Cycle 6 of the Canadian Health Measures Survey, including measurements of bisphenol A (BPA), metals, plasticizers, parabens, perfluoroalkyl substances and pesticides, and adds important new knowledge to our understanding of Canadians' exposure to these chemicals.
Did you know?
Since 2007, average concentrations have declined for many chemicals in the Canadian population as measured through the Canadian Health Measures Survey. In 2021-22, Health Canada published Biomonitoring Fact Sheets for 8 priority chemicals: arsenic, cadmium, lead, mercury, per- and polyfluoroalkyl substances (PFAS), di(2-ethylhexyl) phthalate (DEHP), bisphenol A (BPA), and parabens. The fact sheets summarized key findings from the Survey, including trends in chemical concentrations over time.
The Department also raised public awareness about the health risks of chemicals and pollutants that may be found in and around the home via the Healthy Home Campaign. It continued to provide science-based information to Canadians on chemicals of concern and expanded its reach by investing in projects to accelerate the digital transformation of outreach activities. For example, a digital influencer campaign provided information on asbestos, home renovations, radon, air quality and household chemical safety, reaching some 970,000 Canadians.
Health Canada continued to adapt its traditional outreach materials to enable virtual delivery, including developing Healthy Home webinars and content for virtual tradeshows and presentations. It also developed additional animated videos with "Tips to Protect your Family from Chemicals and Pollutants" and targeted communities with limited or no internet access via a direct mail campaign.
The Department collaborated with community leaders to ensure information reached the most vulnerable Canadians. By training members of the Newcomer Centre of Peel and Carefirst Seniors & Community Services Association to deliver Healthy Home messages, it was able to reach out to newcomers and seniors in multiple languages. All of the participants in these sessions reported they intend to apply the knowledge they gained.
The Environmental Health Action Card (outlining action-based learning activities on environmental health), developed in 2021 to include in the EcoSchools virtual curriculum-linked certification program, was used by 98 schools from 36 school boards/districts across Canada. Of these, 45 schools focussed on the Chemical Safety material. Over 140 hours of related environmental health learning took place, involving over 24,000 students.
In addition, Health Canada issued updated tips and information on various drinking water pollutants – its Drinking Water webpages continued to be among the top 3 most viewed pages related to environmental health on Canada.ca.
Supporting the safety of consumer products and cosmetics
In 2021-22, Health Canada notified Canadians of 233 consumer product and cosmetic recalls, 59 of which were coordinated as joint recalls with the U.S. and/or Mexico.
What's New?
In March 2022, Health Canada and PHAC partnered with the Canadian Paediatric Society and Baby's Breath Canada to promote Canada's first annual Safe Sleep Week. The theme "Safe Sleep All Day and All Night – Night Time, Nap Time, Every Time" encouraged caregivers to remember safe sleep guidelines for every sleep and to establish safe sleep routines.
Babies can fall asleep quickly and in unexpected places, so it's important to remember that products designed for play and travel are not designed for sleep. Babies younger than 4 months are at greatest risk of sleep-related deaths.
To further promote awareness of potentially unsafe consumer products, the Department participated in internationally coordinated consumer product awareness campaigns, including a webinar on cribs, cradles, bassinets and play yards/play pens with the U.S. and Mexico. In addition, it hosted the 5th North America Consumer Product Safety Summit in September 2021, which brought together North American and international product safety regulators and industry stakeholders. The Summit included sessions on e-commerce and COVID-19, safety of seniors, and safety by design. The 3 North American regulators discussed potential future collaborative work, informed by the presentations and engagement.
Health Canada also continued to actively promote awareness of, and compliance with, regulations, while also monitoring progress towards compliance and taking enforcement action as necessary. For example, to support industry in complying with the Corded Window Covering Regulations, the Department worked with interested parties and industry to answer questions and provide additional guidance.
In addition to consulting with stakeholders on proposed amendments to the Surface Coating Materials Regulations, and notifying stakeholders of proposed amendments to the Cosmetic Regulations for certain fragrance allergens in cosmetics, Health Canada published a notice encouraging regulated parties to use safe and non-chemical alternatives to meet consumer product flammability performance requirements. The Department also began updating the Carriages and Strollers Regulations to support innovative product design while enhancing safety.
Protecting Canadians from radiation
As part of the Federal Nuclear Emergency Plan, Health Canada participated in 13 nuclear emergency drills and exercises to verify operational readiness, and identify any opportunities to strengthen response plans and operational arrangements as part of emergency preparedness.
Did you know?
Health Canada is the lead federal department responsible for coordinating the response to a nuclear emergency under the Federal Nuclear Emergency Plan. In 2021-22, the Department led the federal preparation and participation in Exercise Synergy Challenge 2021, at New Brunswick's Point Lepreau Nuclear Generating Station. The exercise was held virtually and tested the coordinated federal and provincial response to a nuclear emergency.
The Department continued to conduct research and develop relevant information and science-based advice for Canadians and stakeholders on the safety of radiation-emitting devices. For example, it conducted a social media campaign on the safety of radiofrequency electromagnetic fields (RF EMF) and 5G technology to promote awareness about health protections for Canadians and to address misinformation. The campaign produced new communications products, including fact sheets that address common questions and concerns surrounding these RF-emitting devices.
In 2021, Health Canada consulted with Canadians and stakeholders on proposed amendments to requirements for lasers under Schedule II of the Radiation Emitting Devices Regulations. The proposed changes would align Canadian requirements with international standards to help protect Canadians from laser radiation hazards. The consultation informed ongoing development of a regulatory proposal targeted for fall 2022.
In early-2022, the Department conducted public opinion research on the attitudes, knowledge and expectations of Canadians about risks from radiation-emitting devices. The results will help identify radiation topics on which the general public would benefit from additional science-based advice.
Strengthening pesticide regulation and transparency
In 2021-22, Health Canada continued to: promote, monitor and enforce compliance with the Pest Control Products Act (PCPA) and its Regulations; and make timely science-based decisions that supported the safe and sustainable use of pesticides in Canada. For example, the Department registered 389 new pesticide products and completed 15 post-market reviews for pesticides currently on the market, providing more flexible and modern options for Canadians while ensuring continued safety through consideration of the latest science.
A subset of the completed post-market reviews this fiscal year included 8 priority pesticide reviews (i.e., large and complex re-evaluations and special reviews), leaving a remaining 10 priority pesticide reviews heading into 2022-23. Completing these large and complex re-evaluations continues to be an ongoing priority as new re-evaluations and special reviews are initiated every year.
Health Canada developed and implemented a comprehensive plan to inform Canadians and regulated parties about products regulated under Interim Order. The Department published the "Buying Pest Control Products Online" fact-sheet to help Canadians recognize and purchase registered pesticides, including ultra-violet (UV) and ozone devices. It co-led a series of bilateral meetings with relevant industry associations, developed an industry fact-sheet specific to UV- and ozone-emitting devices, and issued compliance promotion packages to Canadian manufacturers and institutional users of these devices. These efforts helped prevent the importation, sale, distribution and use of unregistered products.
In addition, the pesticide regulatory program renewal project was incorporated into a broader initiative (the Transformation Agenda) announced by the GOC in August 2021 to undertake better-informed, transparent, accessible, and more responsive pesticide reviews for the 21st century. Key activities are organized under 4 pillars: improved transparency; increased use of real-world data and independent advice to inform pesticide decisions; strengthened human health and environmental protection through modernized pesticide business processes; as well as a targeted review of the PCPA. 2021-22 achievements included:
- Establishing a Transformation Task Force to design, oversee and implement the 4 pillars;
- Launching a transformation web page to communicate with partners and the public;
- Advancing the development of plain language for public summaries of regulatory decisions;
- Reviewing the PCPA to identify potential opportunities for improvement;
- Conducting public consultations related to a targeted review of the PCPA;
- Publishing a call for nominations for the Science Advisory Committee;
- Conducting a needs analysis and identifying priorities for the application of real-world data;
- Developing a plan for system transformation in support of business modernization and increased transparency;
- Advancing the development of a National Water Monitoring Framework for Pesticides.
Departmental Result 5: Canadians make healthy choices
Helping Canadians make healthy choices in their day-to-day lives is an important part of Health Canada's Health Protection and Promotion core responsibility. Over the course of 2021-22, the Department focused on the following priorities, detailed further below: promoting healthy eating; improving food packaging and labelling; ensuring the safety of the Canadian food supply; fostering international collaboration and coordination; taking action on youth vaping and reducing tobacco use; as well as supporting Canadians in making informed decisions about cannabis use through public education, research and surveillance.
Promoting healthy eating
In 2021-2022, Health Canada continued to advance its Healthy Eating Strategy, which aims to curb the rising burden of obesity and chronic disease by making healthier choices easier for all Canadians.
The Department began to gather data about food and beverage advertising in Canada including in restaurants, in recreation centres and with regard to brand-specific advertisements. Health Canada also collaborated with experts to improve its ability to consistently analyse this data. These efforts will inform the Department's approach towards the advertising of certain foods and beverages to children.
The Department continued to promote healthy eating using Canada's food guide, with a focus on increasing food skills. It created new resources, including recipes, videos and articles, and conducted social media campaigns to facilitate the use of the guide, especially amongst children and youth.
What's New?
In March 2022, Health Canada launched a TikTok hashtag challenge – a social media first for the GOC – to encourage teens to improve their food skills and to make healthy snack choices. The #Explore3ingredients TikTok challenge invited teens to show off their favourite 3-ingredient snacks and inspire others to do the same. During the 60-days the challenge was live, ads for it resulted in over 12.3 million impressions (number of times content is displayed to users). Users created 50 of their own videos, resulting in over 740,000 additional views.
Using new child- and youth-centred branding, Health Canada reached out to encourage healthy eating from youth into adulthood, through experiential marketing outreach in schools to raise awareness of the impact of food marketing on healthy eating. This experiential marketing informs Canadians about important health issues through engaging activities. Events are led by one or more ambassadors and can be held in-person or virtually. To pilot test the approach, Canada's food guide virtual classroom sessions featured videos and interactive online activities delivered in schools across the country. These sessions also contributed towards a module for the use of educators. The pilot that took place from March to May 2022, delivered 273 sessions and engaged with 5,173 students.
Recognizing the importance of reaching young people, the Department collaborated with P/Ts and stakeholders to develop draft healthy eating criteria aligned with the food guide for implementation in post-secondary institutions and recreation settings.
Health Canada also prioritized the needs of vulnerable populations – including children and youth, young adults, and families who are experiencing or are at risk of food insecurity. For example, it collaborated with the Guelph Family Health Study and the Food Innovation and Research Studio at George Brown College to develop 30 recipes using affordable, accessible and culturally diverse plant-based protein foods targeted to families with young children. Health Canada also developed 15 recipes for the food guide in collaboration with Quell, an organization dedicated to reducing inequities in the food and hospitality industry and creating opportunities that reflect Canada's diverse and multicultural population.
Did you know?
Health Canada consulted on proposed amendments to align the Table of Daily Values with updated dietary reference intakes for sodium and potassium, both of which are considered essential nutrients of public health concern. It also proposed amendments to the Table of Reference Amounts for Foods based on new data and began to address gaps in certain food categories, with plans to finalise these changes in 2022-23.
Health Canada and several U.S. government agencies jointly commissioned a review of the dietary reference intake values for energy, which were last published in 2002. Work will include assessing human energy intake requirements as well as links to health outcomes, including chronic diseases.
The Department continued to promote safe food handling practices in the lives of Canadians. For example, it distributed and promoted videos (e.g., how to properly chill food to prevent illness), and shared images on social media that demonstrated how to handle and prepare food safely (using the WHO's clean, separate, cook and chill model). Popular Canadian television networks also shared these messages during on-air cooking segments.
To promote sodium reduction in the food service and restaurant sector, Health Canada worked with Colleges and Institutes of Canada to raise awareness about the importance of reducing sodium, particularly in food produced by small, independent restaurants and food-service establishments. It also targeted educational materials to food technology and nutrition students and professionals on how to reduce the use of salt or sodium-based ingredients.
The Department remains committed to consulting on sodium reduction targets for foods sold in restaurants and food services. However, efforts were postponed in 2020-21 given the pandemic's severe impact on the food service industry.
To improve the bone health of Canadians, Health Canada began to implement its vitamin D fortification strategy with a marketing authorization, in January 2022, that permitted manufacturers to increase the amount of vitamin D added to cow's milk, goat's milk (2 µg/100 mL each) and margarine (26 µg/100 g). Further changes allowed increased vitamin D levels in fortified plant-based beverages. The final planned step, permitting the vitamin D fortification of yogurt, is expected in 2022-23.
Improving food packaging and labelling
Health Canada continued to develop new regulations aimed at introducing a mandatory front-of-package nutrition symbol on prepackaged foods with high levels of saturated fat, sugars and/or sodium. Once implemented, this new, easy-to-identify symbol will help consumers make more informed and healthier food choices.
Health Canada coordinated with CFIA to establish a standardized cycle for implementing regulations that require a change to food labels. This policy, which took effect in August 2021, provides greater predictability and transparency for the food industry around the timing for implementing label changes. This transparency will help the industry to plan accordingly and reduce associated costs.
Ensuring the safety of the Canadian food supply
In 2021-22, Health Canada launched a collaboration with national and international partners to address the issue of food fraud. Misrepresenting the contents of a food product to consumers can result in health risks and safety issues (e.g., if unidentified allergens or hazardous materials are added to food products). To address the challenge of food fraud both nationally and internationally, the Department collaborated with the CFIA, the U.S. FDA, and the U.S. Pharmacopeia to convene the first of a series of Food Fraud Information Exchange meetings.
What's New?
In an effort to prevent shortages of infant formulas, human milk fortifiers and metabolic products in Canada due to the temporary closure of a large American manufacturing plant, Health Canada recommended that CFIA exercise enforcement discretion with respect to certain labelling and composition requirements on importing similar products from trusted jurisdictions (such as the U.S. and E.U.). As a result, Canadians that rely on these products were assured of a safe and reliable supply.
Fostering international collaboration and coordination
Health Canada actively contributed to developing international standards for food safety and nutrition through its co-leadership, with CFIA, of Canada's participation at the Codex Alimentarius Commission.
The Department participated in 12 formal Codex sessions and attended multiple special meetings to consider the ongoing impact of the pandemic. It successfully hosted the 46th Codex Committee on Food Labeling, attended virtually by more than 600 representatives from more than 75 countries. The session focused on food labelling – in particular, labeling of allergens, front-of package labelling, e-commerce and the use-of technology in labelling. Health Canada also participated in developing many international food safety and nutrition standards which will help to maintain the safety of the food system in Canada and abroad.
The Department improved its understanding of current and emerging issues, and collaborated in areas related to food pathogens, food chemicals, and nutritional safety. For example, the Department served as secretariat of the International Food Chemical Safety Liaison Group, a forum for discussion and collaboration between government organizations involved in the risk assessment, risk management, and communication of food chemical safety issues.
In addition, Health Canada played a leadership role in developing aspects of a new Food Safety Risk Communication Framework and associated guidelines as part of the Asia Pacific Economic Cooperation (APEC) project, "Trade facilitation through the Development of an APEC Food Safety Risk Communication Framework". The Framework recommends international best practices in effective food safety risk communication and serves as a guide to regulatory agencies during food safety incidents or emergency situations, as well as in everyday food safety risk communication scenarios. It also provides guidance to the food industry regarding food safety risk communication.
In early 2021-22, Health Canada shared for consultation proposed new guidelines for plant breeding, based on scientific data gathered from international and domestic stakeholders. Final guidelines will be published in 2022-23.
Health Canada continued to develop a formal process to share safety assessment information with Food Standards Australia New Zealand. This collaboration will help reduce duplication and regulatory assessment costs for food producers, and streamline the food approval process.
The Department continued to work with other compatible regulators to develop international regulations for novel foods and feeds and to establish microbiological standards and detection methods for all parts of the food chain (including primary production, feed products, food production and handling).
Taking action on youth vaping and reducing tobacco use
Health Canada remained concerned about the levels at which Canadian youth use vaping products and took additional steps over the past year to protect a new generation from the risk of nicotine addiction and other vaping-related harms. At the same time, the Department continued to implement Canada's Tobacco Strategy - a modernized approach for successful tobacco control with the goal of reducing tobacco use to less than 5% by 2035.
Health Canada launched the first legislative review of the Tobacco and Vaping Products Act, including a public consultation to gather the perspectives of Canadians, experts, and other stakeholders with a particular emphasis on the Act's ability to address youth vaping. The Department will examine the early evidence from the Act's first 3 years to assess the progress made towards achieving its vaping-related objectives, and report to Parliament in 2022-23.
Reducing youth vaping
In 2021-22, in addition to publishing the Nicotine Concentration in Vaping Products Regulations, Health Canada pre-published the proposed Order Amending Schedules 2 and 3 to the Tobacco and Vaping Products Act (Flavours) and Standards for Vaping Products' Sensory Attributes Regulations in the Canada Gazette, Part I for a 75-day public consultation period. Restricting vaping product flavours is expected to further reduce the appeal to youth while still leaving some flavour options available for adults who smoke and wish to transition to vaping as a less harmful source of nicotine. The Department received over 25,000 submissions during the consultation.
What's New?
In 2021-22, Health Canada continued to work with key stakeholders to respond to youth vaping. The Department finalized and published the Nicotine Concentration in Vaping Products Regulations in the Canada Gazette, Part II, which set a maximum nicotine concentration of 20 mg/mL for vaping products marketed in Canada. This measure is expected to help reduce the appeal of vaping products to youth.
In addition, Health Canada continued the youth vaping prevention campaign – "Consider the Consequences of Vaping" – to reach youth (13 to 18 years of age) and their parents. The campaign aimed to discourage youth from taking up vaping by educating them about the risks and harms; providing parents, adults, and educators with resources to support conversations with youth; and increasing awareness of where to get more information.
Other significant vaping-related activities in 2021-22 included:
- Sampling over 200 vaping products and applying laboratory methods to determine their nicotine concentrations as a part of the Department's ongoing inspection program;
- Inspecting 76 Canadian vaping product websites to determine if the sellers had instituted appropriate mechanisms to prevent youth from accessing vaping product advertising or promotions;
- Inspecting 1,320 gas and convenience stores and 191 specialty vaping outlets to assess compliance with product packaging, labelling, promotion, and nicotine concentration requirements;
- Assessing vaping products sampled from manufacturers with a focus on packaging, labelling, and nicotine concentration. Health Canada reviewed 150 vaping product labels and laboratory-tested 190 samples for nicotine concentration;
- Publishing the Department's third compliance enforcement report outlining the results of a series of online inspections of vaping product promotions.
Reducing tobacco use
In 2021-22, Health Canada conducted inspections for tobacco products regarding packaging and labelling requirements during its onsite regulatory visits at 1,320 gas and convenience stores. In addition, it conducted 4 onsite inspections of tobacco manufacturers resulting in the laboratory-testing of 66 samples for cigarette ignition propensity, and the assessment of 126 package/labels to verify compliance with requirements under the Tobacco Products Regulations – Plain and Standardized Appearance.
The Department continued its collaborative work with P/Ts to reduce tobacco use, providing up to $2.5 million to support smoking cessation services via the Pan-Canadian Quitline Initiative. This initiative consolidates cessation services from each P/T, served by independent service providers across Canada, to a single phone number and web address displayed on tobacco product packages.
Working with the Standards Council of Canada and the University of Ottawa Heart Institute, Health Canada advanced the development of voluntary smoking cessation standards for health care organizations. A February 2022 workshop drew some 60 participants, including front-line workers, government representatives, academics and experts, to inform the standards development process.
The Department continued to develop regulations to renew and strengthen existing regulatory requirements for tobacco product package labelling. This work involved exploring updates to the health-related messages (including warnings, health information, and toxicity information) that must be displayed on tobacco products packaging to reduce their appeal.
The Canadian Tobacco and Nicotine Survey conducted by Statistics Canada on behalf of Health Canada was updated to double the number of youth and young adults sampled and to provide provincial level reporting of vaping product use. The flagship Canadian Community Health Survey reported on vaping at the national level for the first time. This will allow for detailed sociodemographic analysis of Canadians who vape and/or smoke.
In 2021-22, Health Canada's SUAP provided $30,000 in grants and $3.5 million in contribution agreements for 9 projects focused on prevention, protection and/or cessation for both tobacco and vaping products.
Supporting Canadians in making informed decisions about cannabis use through public education, research and surveillance
Health Canada monitored changes in knowledge, attitudes and behaviours on cannabis through the 2021 Canadian Cannabis Survey as well as the 2019 Canadian Alcohol and Drugs Survey. The Department also adapted its public education and awareness initiatives to address knowledge gaps identified by research and surveillance to help ensure that Canadians were provided with relevant information related to cannabis use and its associated health risks.
What's New?
As one example of the 33 cannabis-related projects funded by SUAP, the Canadian Nurses Association received $1.2 million to develop and disseminate a national nursing framework on the legalization of cannabis that helped nurses interact directly with populations at a high risk for cannabis harms. The framework provided learning tools such as evidence-based modules, short videos (including an animation of how cannabis affects the brain) and a stigma-busting poster to guide interactions with those who use substances. Over 500 nurses participated in these learning opportunities, with 100% positive feedback.
Health Canada delivered evidence-based and innovative public awareness initiatives, including proactive monthly social media content, which provided thematically relevant and consistent information on the health and safety of cannabis use.
In March 2022, the Department launched the redesigned Pursue Your Passion campaign. The teacher-led presentation educates youth aged 13 to 15 about the physical and mental health effects of cannabis. It also issued a new installment of the Cannabis Resource Series, a set of public education resources designed to provide Canadians with additional health and safety information related to cannabis. The health effects of cannabis on adults over 55 piece was published on Canada.ca and promoted widely to stakeholders and partners.
Ongoing support was provided by Health Canada to community-based and Indigenous organizations to educate communities on the health effects of cannabis use. In 2021-22, SUAP invested over $12 million in contribution agreements to fund 33 projects related to the use of cannabis with a focus on youth and young adults, Indigenous populations, health professionals and educators, and other at-risk groups. A total of 2,600 cannabis-related knowledge products/learning opportunities were delivered and accessed approximately 74 million times. Health Canada also launched the final year of the microgrants program, which delivered education and awareness to youth and young adults about the health effects and risks of cannabis and vaping.
Recognizing that consistent research is fundamental to understanding the effects of cannabis use, the Department undertook projects to begin addressing information gaps, such as the potential health hazards associated with inhalation of cannabis-derived vaping aerosol. The data gathered will contribute to informing regulatory and compliance efforts, risk assessment, and public education and awareness.
In addition, Health Canada continued to: monitor the emerging scientific literature on the risks, harms and benefits of cannabis; provide scientific advice; conduct health risk assessments and other risk activities; conduct research; as well as monitor, assess, and communicate about cannabis adverse reactions, including vaping-associated lung injury. The Department conducted 3 health risk assessments, over 250 risk-related consultations/requests, and screened 142 unique cases of adverse reactions associated with cannabis products (20% of which required hospitalization).
In 2021-22, SUAP directed $3 million in cannabis funding to MHCC to support both current and future research, further build an evidence-base, and mobilize knowledge regarding the impact of cannabis use on mental health in a legalized and regulated environment.
In addition to the $11 million directed to CCSA to address substance use in Canada, Health Canada provided $2.3 million to the organization specifically for cannabis research to build evidence needed to inform policy development, including the following initiatives:
- Second-year funding of 5 Partnership for Cannabis Policy Evaluation Grants to gather evidence on the impact of different regulatory approaches on key health, social and public safety domains, as well as fostering collaboration across jurisdictions;
- Second-year funding of 18 grants for cannabis research projects under the heading of "Closing the Gaps in Cannabis Research";
- 3 new grants for cannabis research projects under the heading of "Closing More Gaps in Cannabis Research".
In order to continue building knowledge and closing gaps, CCSA released a range of products in 2021-22 related to: home cultivation trends and associated risk behaviors in Canada; unintentional pediatric cannabis exposures; youth interactions with the justice system post-legalization of cannabis; as well as cannabis-related emergency department visits and hospitalizations. In addition, the organization expanded its efforts on stigma reduction and training, targeting the law enforcement community, as well as public awareness of cannabis use and mental health.
Key risk for Core Responsibility 2: Health Protection and Promotion
Information on Key Risks is available on Health Canada's website.
Results for Core Responsibility 2: Health Protection and Promotion
The following tables show, for Core Responsibility 2: Health Protection and Promotion, the results achieved, the performance indicators, the targets and the target dates for 2021–22, and the actual results for the three most recent fiscal years for which these results are available.
| Departmental result indicators | Target | Date to achieve target | Actual results |
|---|---|---|---|
| Percentage of new drug decisions issued within service standardsTable dr3 Footnote 1 (Baseline: 88% in 2017-2018) |
At least 93% | March 31, 2022 |
|
| Percentage of Risk Management Plan reviews for new drug decisions completed within service standardsTable dr3 Footnote 2 (Baseline: 91% in 2017-18) |
At least 90% | March 31, 2022 |
|
| Percentage of drug companies deemed to be compliant with manufacturing requirements under the Food and Drugs Act and associated regulations | Between 85% and 95%Table dr3 Footnote 3 | March 31, 2022 |
|
|
|||
| Departmental result indicators | Target | Date to achieve target | Actual results |
|---|---|---|---|
| Percentage of domestic consumer product recalls communicated to Canadians in a timely manner (Baseline: 86% in 2016-17) |
At least 90% | March 31, 2022 |
|
| Percentage of actions taken in a timely manner to protect the health of Canadians from substances found to be a risk to human health (Baseline: 85% in 2016-17) |
Exactly 100% | March 31, 2022 |
|
| Percentage of pre-market submission reviews that are completed within service standards (Baseline: 95% in 2019-20) |
At least 90% | March 31, 2022 |
|
|
|||
| Departmental result indicators | Target | Date to achieve target | Actual results |
|---|---|---|---|
| Percentage of Canadians (aged 15+) who are current cigarette smokers (Baseline: 16% in 2017) |
At most 5% | March 31, 2035 |
|
| Percentage of Canadians (aged 15-24) who have used cannabis in the last 12 months (Baseline: 26.9% in 2018-19 [14.2% of Canadians aged 15-17 and 31.4% of Canadians aged 18-24]) |
At most 29%Table dr5 Footnote 4 | March 31, 2025 |
|
| Percentage of Canadians who use dietary guidance provided by Health Canada (Baseline: 41% in 2012) |
At least 50% | March 31, 2022 |
|
|
|||
Budgetary financial resources (dollars) for Core Responsibility 2: Health Protection and Promotion
The following table shows, for Core Responsibility 2: Health Protection and Promotion, budgetary spending for 2021–22, as well as actual spending for that year.
| 2021–22 main estimates |
2021–22 planned spending |
2021–22 total authorities available for use |
2021–22 actual spending (authorities used) |
2021–22 difference (actual spending minus planned spending) |
|---|---|---|---|---|
| 1,104,086,037 | 1,104,086,037 | 986,642,487 | 787,250,023 | -316,836,014 |
Note: The variance of $316.8 million between actual and planned spending is mainly due to a transfer to PHAC for procurement of COVID-19 tests; as well as a repurpose and reprofile of funds related to the creation of a critical drug reserve. |
||||
Human resources (full-time equivalents) for Core Responsibility 2: Health Protection and Promotion
The following table shows, in full-time equivalents, the human resources the Department needed to fulfill this core responsibility for 2021–22.
| 2021–22 planned full-time equivalents |
2021–22 actual full-time equivalents |
2021–22 difference (actual full-time equivalents minus planned full-time equivalents) |
|---|---|---|
| 5,933 | 6,527 | 594 |
Note: The variance in full-time equivalents utilization is mainly due to additional in-year resources received for continuing Canada's chemical management regime; regulatory and operational functions to support critical COVID-19 focused operations; addressing the opioid overdose crisis and problematic substance use; as well as ensuring the ongoing integrity of the Public Service Occupational Health Program. |
||
Financial, human resources and performance information for Health Canada's Program Inventory is available in GC InfoBase.
Internal Services
Description
Internal services are those groups of related activities and resources that the federal government considers to be services in support of programs and/or required to meet corporate obligations of an organization. Internal services refers to the activities and resources of the 10 distinct service categories that support program delivery in the organization, regardless of the internal services delivery model in a department.
The 10 service categories are: Management and Oversight Services; Communications Services; Legal Services; Human Resources Management Services; Financial Management Services; Information Management Services; Information Technology Services; Real Property Management Services; Materiel Management Services; and Acquisition Management Services.
Results
Health Canada's greatest strength is an engaged, empowered and well-equipped workforce with employees who have the competencies (including science and regulatory skill sets), tools and opportunities to succeed in the pursuit of excellence in program and service delivery.
The Clerk of the Privy Council noted in his letter to the Prime Minister in the Twenty-Eighth Annual Report on the Public Service of Canada, that this is a period characterized by the COVID-19 pandemic and Canada's confrontation with racism and intolerance. It has presented many opportunities to serve in different ways and to adapt as a Public Service. We must take action to be better, more just, open, and inclusive, particularly by tackling systemic racism and advancing work on diversity and inclusion in our organizations. Health Canada continues to support the government-wide goals of Public Service Renewal, through initiatives that foster inclusiveness, agility and resilience.
The Department remains committed to ensuring that all employees feel safe and are treated with respect, dignity and fairness in the workplace, including instilling positive values and tackling racial bias, harassment and discrimination. Health Canada also remains committed to eliminating barriers to success and career progression through more inclusive recruitment practices.
In 2021-22, the Department focused on the following priorities:
Building a healthy, diverse and inclusive workforce
Health Canada is committed to ensuring a workplace that is free of racism and discrimination, and where all employees feel safe and are treated with respect, dignity and fairness. These values are the foundation of who we are, what we do, and how we do our work.
Did you know?
In 2021, Health Canada was recognized as one of Canada's Best Diversity Employers (for the 7th year running), one of Canada's Top Employers of Young People (for the 11th year running), and for the second time, as one of Canada's Top 100 Employers, which recognizes organizations with exceptional human resources programs and forward-thinking workplace policies. The Department was recognized for its continuous support to employees during the COVID-19 pandemic and its ongoing commitment to eradicating systemic racism and discrimination from our workplace culture.
In 2021-22, the Department continued to advance its Multi-Year Diversity and Employment Equity Plan:
- Working to instill policies and activities that foster an inclusive, equitable and accessible workplace via the Leadership Council on Diversity;
- Launching initiatives such as Mentorship Plus and the Equitable Access to Language Training Program as a means to address systemic barriers;
- Supporting managers and employees with accommodation needs and promoting the use of the GOC Workplace Accessibility Passport, via the Workplace Wellness Service Centre;
- Fostering a learning culture, including the Indigenous Learning Series and sessions on unconscious bias/anti-racism and on the prevention of harassment and violence in the workplace.
A horizontal audit conducted by the Canadian Human Rights Commission under the Employment Equity Act (EEA) acknowledged that the Department has taken significant and meaningful steps to establish goals and monitor progress regarding diversity and inclusion. Other initiatives that helped foster a workplace free of racism and discrimination, included:
- Continuing to review internal policies, procedures and practices from an employment equity perspective and filtering the results into the Department's strategic planning;
- Leveraging the newly created Leadership Council on Diversity and Inclusion and Employee Networks to combat racism and discrimination;
- Developing an action plan to address findings from internal anti-racism listening sessions and implementing concrete actions to create a more diverse and inclusive workplace;
- Implementing a Safe Space Initiative within the independent Centre for Ombuds, Resolution, and Ethics to support diverse groups, including Black, Indigenous, racialized, people with disabilities and 2SLGTBQI+, facing discrimination in the workplace.
Initiatives that helped attract and retain a diverse, inclusive, and bilingual workforce within a healthy, accessible and high-performing workplace, included:
- Continuing to implement mental health and wellness strategies for employees and updating resources, including the National Standard for Psychological Health and Safety in the Workplace;
- Streamlining access to updated mental health resources through creation of the My Mental Health desktop icon and the Mental Health Events Calendar as well as launching the Santé podcast;
- Identifying Accessibility Ambassadors and beginning to train them to provide expert advice related to accessibility/duty to accommodate in the staffing context;
- Updating the virtual onboarding program for new employees and adapting Official Language training and Second Language Evaluations for remote learning and testing;
- Leveraging the Persons with Disabilities Network, to inform the development of Health Canada's Accessibility Plan (to be launched in December 2022);
- Holding anti-racism listening sessions to identify discrimination issues and offering specialized counselling support for employees;
- Increasing the number of Black counsellors in the Department's network of mental health professionals (20 added in 2021-22).
Did you know?
In 2021-22, Health Canada delivered training and information sessions to support and promote safe and respectful workplaces and business operations. Topics included diversity and inclusion, anti-racism and discrimination, official languages, and accessibility.
Employee Engagement
Health Canada regularly: engaged employees and sought feedback to evaluate and adjust communications strategies to keep these useful and relevant; checked in virtually on how the pandemic was affecting their professional and personal lives; and shared information and resources to protect their mental and physical health and to support their work and productivity.
Did you know?
In January 2022, Health Canada launched its Mentorship Plus program to further encourage and support marginalized voices, and help eliminate systemic barriers to recruitment, retention and training. The program was specifically designed to support leadership development with a focus on members of underrepresented groups who aspire to leadership and executive positions.
These efforts were integral to supporting the workforce as employees continued to work remotely and to develop plans for re-entry. The Department used a variety of engagement tools, platforms, and fora, including: new web-based channels, Town Hall meetings; targeted presentations, technical briefings for managers, and employee networks; Fire Side Chats on the future of work; as well as employee questionnaires and surveys.
Did you know?
Over the course of the pandemic, integrated digital collaboration tools have become essential to everyday business functions for Health Canada employees. An average user on the Microsoft 365 collaboration platform in any given month takes part in 444 chats, 22 meetings and 40 hours of audio/video calling.
Internal Services continued to support the Department in response to the unique technological, financial and human resources needs created by the pandemic. Support was provided to the workforce, the workplace and the work itself so that Health Canada could continue to deliver on its mandate, whether employees were working remotely or on site.
In 2021-22, the Department: developed and shared occupational health guidance for federal employees in response to changing conditions, including support for workplace re-entry; responded to over 270 inquiries from Federal departments related to occupational health and safety; developed the COVID-19 Vaccine Fundamentals training session delivered by the Canada School of Public Service; implemented simplified and expedited staffing processes to support COVID-19 roles, including large-scale collective staffing processes; and supported employees working remotely through activities such as new Virtual Ergonomic Assessments.
Health Canada continued to foster a culture of care, working through channels such as customized training, leadership coaching, change management and team building. These services reached more than 1,800 employees. In addition, more than 10,000 hours of specialized clinical support, impacting some 2,000 staff, was delivered at ports of entry and quarantine sites, with mental health practitioners stationed full-time at the Vancouver, Montreal and Toronto airports.
To enable onsite presence both during and post-pandemic, Health Canada's workplace re-entry exercise identified employees with an operational need or a personal preference to work onsite at Department locations nation-wide. COVID-19 case monitoring and reporting guidelines for employees working both onsite and remotely, coupled with enhanced cleaning protocols in all Health Canada buildings, helped to ensure a healthy and safe workplace. The Department established a Rapid Testing Program at several onsite facilities, secured additional bandwidth to support remote work, and introduced additional functionality to existing collaboration tools (e.g., MS Teams). Going forward, Health Canada will adjust its approach as required based upon a fact-based risk assessment and support future of work planning.
Did you know?
Health Canada undertook a campaign in July and August 2021 to increase awareness of the availability of free rapid test kits for workplace screening and to encourage small businesses to order kits for their employees. It included an infographic to help employees understand how workplace testing works and why it is important, a handout for pharmacies to distribute to neighbouring businesses, and a variety of digital assets.
Promotional activities included digital advertising, articles and banners/images in other GOC e-newsletters, emails, a push notification through the Canadian Business App, and web buttons at Canada.ca. Overall, materials were seen up to 15.65 million times, resulting in over 126,000 clicks to the "Get free rapid tests" webpage on Canada.ca, and 6-times increase in completed forms to request rapid test kits for workplaces.
Modernizing the workplace to enable a safe and productive workforce with access to modern tools and facilities
In 2021-22, Health Canada continued its efforts to modernize the workplace to enable a safe and productive workforce with access to up-to-date tools and facilities, by:
- Creating a new Digital Transformation Branch to serve Health Canada andPHAC, integrating resources across both organizations while continuing to prioritize many COVID-related activities, and continuing to partner with Shared Services Canada to upgrade and improve network infrastructure;
- Identifying the key components required for the transition to a new departmental financial and material system (SAP S/4HANA) and contracting for an independent assessment of how to best proceed. Given the complexity involved, the implementation timeline has been extended from 2025 to 2027;
- Implementing year 4 of the 2018-23 National Accommodation Strategy to modernize office facilities to specific program requirements, delivering 7 projects across 3 provinces and 4 cities, valued at $7.1 million;
- As part of the Department's ongoing laboratory modernization, delivering the multi-year program of capital and repair projects across its laboratory portfolio to replace and repair aging buildings and improve functionality for science programs.
What's New?
In 2021, Health Canada began to identify areas of improvement and existing strengths in the organization's ethical framework. Once completed in 2022-23, this work will inform program improvements and updates to the Health Canada Code of Values and Ethics.
The Center for Ombuds, Resolution, and Ethics (CORE) offers safe spaces for employees, managers, and executives to raise and address work-related issues at both the individual and organisational levels. It serves as an early warning system for systemic issues and trends, and develops recommendations for those with the authority to act. Contributing to a culture of prevention and ensuring a psychologically-healthy workplace for all, CORE continued to help individuals gain new competencies through such services as coaching, mediation, and value and ethics guidance. Recognising the impact managers have on the health of an organisation, and the acute stresses they have faced, CORE launched a new program in 2021 focusing on emotional intelligence to increase awareness, evaluate existing competencies, and enhance skills through personalized coaching.
Communication Services
Health Canada continued to engage Canadians with the timely and relevant information they needed to take action on their personal and collective health and safety. This was accomplished through a range of traditional, digital and innovative communication strategies and channels that also supported the Ministers in delivering on GOC priorities, including daily postings on Health Canada's social media accounts, web content, digital and traditional advertising, experiential virtual events and partnerships, in addition to more traditional tools such as news releases and proactive media relations.
As described throughout Departmental Results 3-5 earlier in this report, Health Canada's public awareness campaigns informed Canadians about continuing priorities such as: mental health; virtual care and LTC; opioids; cannabis; vaping; tobacco; healthy eating and food safety; as well as environmental health. The Department also launched a revitalised Recalls and Safety Alerts web portal, making it easier for Canadians to find important recalls, health advisories and bulletins.
Providing timely, trusted, and evidence-based information during the pandemic
As Canada's response to the COVID-19 pandemic evolved over 2021-22, Health Canada continued to deliver the most timely, trusted, accessible, and evidence-based information possible to health care providers, stakeholders and Canadians, allowing them to protect themselves, their families, their communities and their businesses. The Department used various communication channels and coordinated with P/Ts to provide the latest updates on public health information, vaccines and treatment, rapid testing, and travel.
Public Health Information
- Delivered public education and advertising campaigns to raise awareness of free mental health and substance use resources and services on Canada.ca;
- Provided COVID-related information in multiple languages via a dedicated toll-free phone service, 7 days a week from 7:00 am-12:00 am. Operational since January 2020, the service received more than 996,000 calls up to end of March 2022, of which 332 callers requested interpretation services;
- Collaborated closely with PHAC to publish and amplify COVID-related data;
- By end of fiscal year, Health Canada's COVID Alert app had been adopted by 9 P/Ts and downloaded over 6.49 million times. No additional promotional activities occurred in 2021-22.
Vaccines and Treatment
- Maintained an online portal with detailed regulatory information about authorized COVID-19 vaccines and treatments;
- Collaborated with PHAC to publish weekly summaries of reports of Adverse Events Following Immunization received for all COVID-19 vaccines.
Rapid Testing
- Provided updated and timely information on how to access free rapid tests; on the importance of rapid testing as a public health measure and tool; and to raise awareness of the distribution of over 402 million rapid tests to P/Ts;
- Coordinated with P/Ts on communications activities surrounding test kit distribution and on raising awareness around the importance of rapid testing;
- Equipped the Canadian Red Cross with social media, news releases and messaging for their outreach activities aimed at vulnerable populations.
Travel
- Expanded the Canada.ca/coronavirus web site to enable Canadians to access all federal COVID-related information and programs from a single site;
- Updated information on changing border measures and delivered public education and advertising campaigns to ensure travellers were aware of travel requirements;
- Worked with Global Affairs Canada to provide easy to follow, up-to-date, border and travel health advice for people coming to Canada;
- Produced a digital engagement kit and marketing toolkits on border measures that stakeholders such as industry associations used to provide accurate information to their clients/audiences.
Mental Health
Health Canada leveraged multiple platforms to target a diverse range of Canadians, in promoting the Wellness Together Canada portal and its companion app, PocketWell. Communications aligned with key dates, such as Mental Health Week and included robust social media plans, including GIFs, videos and a variety of promotional announcements.
Opioids
The Department implemented a digital campaign to counteract the stigma surrounding those who use substances and inform Canadians about the Good Samaritan law, resulting in some 42.4 million impressions and 12.8 million completed video views. It also launched the Stigma Gallery – a hub of videos, quotes and personal stories that put a human face on this issue – as well as delivered 540 virtual sessions to high school classes about harms associated with opioid use and the importance of reducing stigma.
Cannabis
Over the past fiscal year, Health Canada launched several initiatives to help communicate public education messaging about the health impacts of cannabis, particularly focused on youth. In May 2021, the Department hosted its 4th national symposium to discuss gaps in public education and awareness as well as the intersection between the COVID-19 pandemic and substance use.
Vaping
Health Canada continued to educate youth about the risks and harms of vaping through its "Consider the Consequences of Vaping" campaign, as well as provide parents, adults, and educators with resources to support conversations with youth. The Department hosted over 721 virtual events in schools across the country reaching approximately 18,000 students and posted digital advertisements that generated 43 million impressions, 19 million complete video views and 162,000 clicks to the website.
Tobacco
The Department launched a digital advertising campaign aimed at improving the rate of quitting among adults, resulting in 360,000 impressions and 27,600 clicks to the website. This campaign targeted adult smokers aged 35-64, as these older adults have higher rates of smoking.
Healthy Eating and Food Safety
Health Canada delivered a marketing campaign aiming to raise awareness among youth of Canada's food guide and help improve their food skills, while incorporating safe food handling practices as norms in everyday life. Videos and images provided basic tips on handling and preparing food safely; television networks featured messages during on-air cooking segments; and a TikTok video encouraged youth to share 3-ingredient snack recipe videos. Over 49 videos were posted, generating over 740,000 views.
Did you know?
Health Canada's Know More Opioids campaign has engaged over 168,000 youth and young adults across Canada since its launch in 2018, with 1,050 visits to high schools (in-person and virtually) and 43 events/festivals. The website, launched in March 2019, features resources, activities and awareness products related to opioid use, harms and stigma and has now been connected to the Health Canada Experiences portal for all of Health Canada's youth and young adult-focused marketing campaigns.
Environmental Health
The Department supported the renewal of the Chemicals Management Plan and the release of various air quality research and awareness initiatives, such as the Subway Air Quality Initiative and promoting the Air Quality Health Index. Health Canada also worked closely with ECCC on joint files to ensure coordinated communications, including:
- Firefighters Action Plan (flame retardants);
- Renewal of the CEPA;
- Launching consultations on mandatory labelling for chemicals in consumer products;
- Supporting the release of Health of Canadians in a changing climate: advancing our knowledge for action.
The Department reinforced its Healthy Home marketing campaign by featuring 20 Canadian social media influencers who delivered messages about the importance of ventilation, radon testing and chemical safety in the home. Health Canada also maintained social media efforts for wildfire smoke, extreme heat, as well as water and air quality.
Budgetary financial resources (dollars) for Internal Services
The following table shows, for internal services, budgetary spending for 2021–22, as well as actual spending for that year.
| 2021–22 main estimates |
2021–22 planned spending |
2021–22 total authorities available for use |
2021–22 actual spending (authorities used) |
2021–22 difference (actual spending minus planned spending) |
|---|---|---|---|---|
| 301,904,724 | 301,904,724 | 555,219,990 | 513,234,110 | 211,329,386 |
Note: The variance of $211.3 million between actual and planned spending is mainly due to additional funding for the operating and capital budget carry forwards of which a portion was set aside to support strategic investments in 2022-23; the reallocation of resources to meet program needs and priorities; regulatory and operational functions to support critical COVID-19 focused operations; as well as internal services resources received from various Treasury Board approved initiatives. |
||||
Human resources (full-time equivalents) for Internal Services
The following table shows, in full-time equivalents, the human resources the department needed to carry out its internal services for 2021–22.
| 2021–22 planned full-time equivalents |
2021–22 actual full-time equivalents |
2021–22 difference (actual full-time equivalents minus planned full-time equivalents) |
|---|---|---|
| 1,804 | 2,573 | 769 |
Note: The variance in full-time equivalent utilization is mainly due to a technical adjustment for the provision of shared services to PHAC; as well as additional resources received in-year for the internal support services from various Treasury Board approved initiatives. |
||
Experimentation
Fostering innovation and experimentation (I&E) at Health Canada continues to be a departmental priority. A thoughtful and deliberate approach to I&E allows us to discover new insights, technologies and processes, which improve our workplace and deliver better outcomes for Canadians.
In 2021-22, the Department advanced the creative capacity for its employees by promoting its Innovation and Experimentation Policy Framework, hosting bi-monthly Mindset Matters webinar series for learning and sharing, and continued investments in I&E projects and the sharing of learnings and results through various on GOC platforms and communication forums.
Now in its fourth year, Health Canada's Solutions fund continues to provide a space for employees to test ideas and generate evidence to inform decision-making. Since the launch of the Fund in 2018, the Department has supported 17 projects. Achievements in 2021-22 included:
- Project Cipher demonstrated that the Department can prepare compliance and enforcement inspection reports faster by applying machine learning. Results of a pilot project are expected in 2022-23. This new approach is supported by the Canada School of Public Service and is being considered by various other government departments with a regulatory mandate.
- Project Hummingbird tested the value of using satellite and drone imagery to prepare inspection reports of outdoor cannabis license holders and industrial hemp cultivators. Results are promising and inspectors are piloting this new approach, with a final report expected in 2022-23.
- Project PRODigy applied user experience design to revamp the existing consumer product incident-reporting portal. Increased reporting reduces data gaps and helps Health Canada identify dangerous products. The Department is now testing to see if consumers are more likely to submit consumer product incidents using the new form. If increased completion and submission rates are observed, it will be officially introduced in early 2022-23.
- Project Kelpie tested the possibility of using social media and web monitoring tools to aggregate content across social media platforms and identify potentially non-compliant promotion of tobacco and vaping products to youth. Results were positive and Health Canada expects to test the integration of these tools within its existing compliance and enforcement framework in 2022-23.
- Project Fourier-Transform Infrared Spectroscopy (FTIR) developed a new technique to identify foreign matter in health and cannabis products using FTIR spectroscopy. The experiment demonstrated that foreign matter could be identified within a few days, a significant reduction from the current three-week timeline. Health Canada will be looking at expanding this process at other Health Canada labs in 2022-23.
- Project Cognit.io explored the use of a human-centric, Artificial Intelligence (AI)-assisted engine to support the accuracy, consistency, and speed of assessing complex natural health products. A proof of concept is being developed which aims to test and evaluate the AI-assisted engine's output. Scientific evaluators will validate the proof of concept in 2022-23.
- Project Nitro explored the use of Robotic Process Automation to increase efficiency and prevent user errors when processing human resource transactions. The benefits include the ability to: meet service obligations; reduce HR backlogs; improve pay stabilization (i.e., timely entry of HR actions that impact pay); enhance data quality; and reduced errors. Health Canada has begun to test this approach, with results expected towards the end of 2022-23.
- Project Apollo explored the feasibility of using game-based learning to teach Canadian youth about potential environmental health risks related to air quality, radon, and chemicals. Health Canada will continue to engage young people in the coming year with the goal of testing a proof of concept in 2022-23.
- Project D.A.T.A. (Data Annotation Training sets for AI Tools) explored using machine-learning algorithms to automatically extract information from scientific documents and literature reviews. This solution could greatly improve the efficiency of scientific review processes. Final results are expected in 2022-23.
- Project LabINT (Laboratory Innovation for Natural Health Products Testing) explored options to provide faster, more rigorous, more relevant laboratory testing services for suspected quality defects in natural health products. This project is ongoing through 2022-23.
- Project Citizen Science explored using a collaborative approach between Health Canada researchers and the public. Users tested guidance needs and digital platform requirements to facilitate its uptake. Pilot projects will be developed in 2022-23 to further test the feasibility of this approach.
Did you know?
After 2 years of study, Health Canada is now pilot testing Project Cyclops – a mobile-friendly application that helps inspectors assess whether natural health products, cosmetics and pesticides should be allowed to enter Canada. If successful, this tool would provide inspectors with instant access to relevant and detailed information for faster decision-making when these products arrive at our borders.
Additional examples of I&E that began or continued in 2021-22 include:
- Project Drab – Health Canada continued developing and testing this digital solution to automate the analysis of tobacco product labels.
- Project Sia – The Department began developing and testing a data visualisation tool that integrates data from different sources by layering them in maps or other means of visualisation. This type of data visualisation aims to inform and improve C&E decision-making.
- Project Fox It – Health Canada is experimenting with a process tool to maximise its efficiency in managing public inquiries. If proven successful, this tool could improve the way the Department responds to inquiries from Canadians.
- Project Eagle Eye – The Department successfully implemented and continues to refine software tools and processes to inspect cannabis-producing facilities virtually. The shift to using virtual inspections where possible is estimated to have reduced the cannabis inspection program's carbon footprint by over 50%.
- Individualized Accommodations Passport – In partnership with Treasury Board Secretariat and others, Health Canada explored ways to replace its existing paper-based Accommodations Passport with an electronic version. The Passport allows employees to share information about adaptive tools or support measures to facilitate recruitment, retention, promotion, and mobility of employees with disabilities. Work will continue in 2022-23.
Under the Innovative Solutions Canada program, Health Canada invested up to $1 million in each of 5 Canadian companies in 2021-22 to develop a "prototype" of their innovation in response to the following health system challenges:
- Point of care diagnostics to combat AMR and help address the rise of related infections;
- Leveraging AI technologies to predict possible organ donation recipient matches and improve donation rates;
- Developing a cost-effective and reliable method to identify microbial mixtures, characterizing their stability, and predicting possible interactions that may mask or enhance adverse effects in humans.
Health Canada also collaborated with the National Research Council and Environment and Climate Change Canada to support 2 projects related to the Innovative Solutions Canada COVID-19 challenges. The projects address the feasibility of compostable disposable surgical masks and respirators, as well as recycling technologies for disposable (single-use) personal protective equipment, used in the Canadian healthcare sector.
Finally, the Department also supported testing of prototypes related to: a peer-to-peer social health platform with curated content; the development of resources and tools to assess physical and mental wellness; and an AI-enabled digital solution to support hospital emergency departments in more efficiently managing surges in patient flows and volumes.
Sex- and Gender-Based Analysis Plus
Health Canada renewed both its Sex- and Gender-Based Analysis (SGBA Plus) Policy and associated Action Plan for another 4 years starting in early 2022. The Plan and Policy aim to strengthen the integration of sex, gender and other identity factors (such as age, race and income level) in the externally and internally facing work of the Department and serve as the main driver for advancing equity and improving approaches to diversity and inclusion.
The plan underscores an intersectional approach to this work. Priorities identified under the renewed Action Plan were established based on the results of a formal evaluation in 2021 and the lessons learned from 2017 to 2021, including those derived over the course of the COVID-19 pandemic. They include:
- Increasing governance, accountability and transparency in the integration of SGBA Plus in the Department's decision-making;
- Strengthening departmental knowledge and capacity to apply SGBA Plus using an intersectional approach;
- Collaborating with internal and external partners to strengthen the Department's sex, gender and diversity-related evidence base and expertise;
- Enabling the collection and use of disaggregated data for rigour in intersectional analysis;
- Enhancing communications, guidelines, tools and resources with clarity on SGBA Plus and intersectionality.
Prior to the renewal, the Department made significant progress in 2020-21 under the more overarching priorities outlined in the initial Action Plan (originally covering 2017 to 2020, and extended through 2021): increasing departmental capacity to apply SGBA Plus; strengthening the sex, gender and diversity-related evidence base and expertise; and increasing accountability for and transparency in implementing SGBA Plus.
Increasing governance and accountability
In 2021-22, Health Canada established a new senior-level accountability structure. This new SGBA Plus Integration Network is facilitating the integration of SGBA Plus into all areas and functions of the Department. Building on this, the revised SGBA Plus Policy outlines responsibilities at all levels to fully integrate SGBA Plus into the work of the Department, while the Action Plan established objectives and expected results for the next 4 years, to be reviewed annually.
Strengthening knowledge and capacity
Health Canada continued to provide training, resources and tools to increase the knowledge and capacity of its employees to integrate sex, gender and diversity into their work. 2021-22 efforts focused on:
- Increasing the understanding of an intersectional approach to SGBA Plus;
- Raising awareness of how SGBA Plus could and should influence the development of policies, programs, regulations, consultations, and guidelines;
- Building a greater understanding of how to apply SGBA Plus to project management and risk communication;
- Integrating SGBA Plus, Anti-Racism, Accessibility and Indigenous lenses in the development and delivery of programs and initiatives.
To further support learning and increase capacity, the Health Canada SGBA Plus Community of Practice was revitalized in 2021-22 to share best practices and approaches for integrating SGBA Plus into the work of the Department.
Employee Assistance Program
Health Canada's Employee Assistance Program provided services to employees in many federal departments and agencies, as well as to members of the Royal Canadian Mounted Police, members of the Canadian Armed Forces, and veterans of these organizations. The Program applies an SGBA Plus lens to its policies, procedures and services. Overall, past research and experience have confirmed that a one-size-fits-all approach is not sufficient to address the wellness needs of a diverse client base. As such, the Program focused on the following priorities throughout 2021-22:
- Optimizing outreach to those who tend to underuse the Program (such as males, 2SLGBTQI+ persons, Indigenous Persons, and victims of intimate partner violence) and/or who might be experiencing increased mental health impacts because of COVID-19 (such as women, Indigenous persons, 2SLGBTQI+ persons);
- Expanding the use of technologies to improve outreach to groups who access services at lower rates by extending availability of the real-time chat service to all client departments on Canada.ca;
- Increasing the overall percentage of mental health professionals available to support clients requesting referral to a counsellor with lived experience or other relevant expertise in supporting a specific community or equity-deserving group;
- Providing specialized training with a clinical focus to the Program counsellor network on supporting 2SLGBTQI+ clients.
Workplace modernization
Health Canada continued to include gender-neutral washrooms in the modernization of its workplace and is diligent in upholding accessible design standards, whenever possible.
Implementing SGBA Plus across Health Canada programs
Health Canada incorporated SGBA Plus into its decision-making process and initiatives, and strove to develop policies and approaches that would address inequities facing at-risk populations.
Health products
In 2021-22, the Department developed a detailed plan with the objective of helping individuals in Canada make better-informed choices regarding their various treatment options, knowing that the safety and efficacy of health products have been tested in clinical trials involving a diverse population. The three main goals of this plan include:
- Improving the quality of SGBA Plus data submitted to Health Canada;
- Improving the manner in which this data is analyzed and reported by Health Canada;
- Increasing the information made available to the public, to build transparency and trust.
Once implemented, the plan is expected to increase knowledge of the safety and efficacy of drugs and medical devices for population groups that previously tended to be underrepresented in clinical trials for these products.
Health Canada also developed new SGBA Plus training specific to scientific experts responsible for reviewing medical devices, based on advice from Health Canada's Scientific Advisory Committee on Health Products for Women. The Committee also provided input on: the draft guidance document "Clinical evidence requirement for medical devices"; a breast implant patient checklist; a triage tool created to prioritize devices that should undergo risk assessment in the medical devices Foresight Project; as well as incorporating SGBA Plus in the Medical Device Incident Reporting Templates with respect to post-market surveillance.
In response to the pandemic, the Department customized a number of post-market surveillance activities for monitoring the safety profile of COVID-19 vaccines and undertook expedited safety assessments that included SGBA Plus, such as:
- Weekly analysis of spontaneous safety reports (in collaboration with PHAC) for adverse events, disaggregated by sex;
- Requiring manufacturers of COVID vaccines to submit risk management plans that include consideration for specific populations;
- Requiring some of these manufacturers to conduct new studies on the effects on pregnant and breastfeeding individuals;
- Requiring manufacturers to submit monthly safety reports that include data stratified by patient characteristics, such as sex.
Health Canada will extend these safeguards for proactive consideration of SGBA Plus beyond COVID-related products. For example, the Department is looking at ways to require disaggregated data on the diversity of clinical trial participants in all drug submissions and to publicly report this data on a regular basis.
In partnership with CIHR's Institute of Gender and Health, Health Canada funded 3 policy-research partnerships between 2018 and 2021 to identify how the Department currently accounts for diversity, sex and gender in the regulation of health products, and to recommend ways to further integrate these concepts. In 2021-22, the Department concluded initiatives with:
- McGill University to investigate consumer perceptions and behaviours in relation to health product labelling for cosmetics, natural health products and non-prescription drugs. This research helped frame a regulatory proposal that improves natural health product labelling, to be finalized in 2022-23;
- The University of British Columbia to analyze the application of SGBA Plus considerations in the prescription drug lifecycle management, and made recommendations for improvement;
- The University Health Network to analyze the application of SGBA Plus considerations in the lifecycle of medical devices. In response, Health Canada developed and is advancing an internal implementation plan focused on guidance, regulations and training.
Compliance and enforcement
The Department integrated accessibility and universal design principles that are reflective of diversity, inclusion and Indigenous perspectives in their training for compliance and enforcement inspectors and analysts.
Other SGBA Plus-focused products for compliance and enforcement developed in 2021-22 included a detailed facilitator-training guide to help support staff training needs, and a self-reflection tool that supports staff interactions with regulated parties during inspections.
Healthy eating policies
All Health Canada nutrition research and surveillance activities included consideration of sex and gender. Depending on the at-risk group under study, it also considered other sociodemographic characteristics such as ethnicity and culture. For example, the Department: considered self-identified sex and age when assessing nutritional adequacy of the population using data from the Canadian Community Health Survey; it considered characteristics related to socioeconomic status (e.g., education, household income) or demographic characteristics (e.g., self-reported race and/or ethnicity) when assessing determinants of nutritional adequacy or status, in addition to sex and gender; when undertaking basic research using animal models, it included male and female animals in the studies where applicable.
Canada's food guide
To promote the use of Canada's food guide, Health Canada continued to develop targeted healthy eating resources and worked with stakeholders to reach individuals in Canada across various settings, ages, and population groups. For example, the Department: created a new, modern, user-centric food-guide recipe gallery that included new culturally-diverse recipes and articles; contracted an organization that represents a diverse group of chefs and food advocates from minority communities to review the guide's content through a multicultural lens and to challenge any assumptions and biases; and continued working with diverse groups of youth and young adults to inform development of new resources and tools, and promote peer-to-peer engagement on healthy eating.
The Nutrition Science Advisory Committee of external experts met 4 times to develop timely and independent scientific and technical advice related to nutrition for the Department. Its advice will be applied to help improve the Evidence Review Cycle approach and to strengthen the monitoring of equity, diversity and inclusion considerations.
Health Canada completed SGBA Plus laboratory research developing genomics as a tool to screen for adverse reproductive and developmental effects of food contaminant chemicals for women compared to men.
Environmental health outreach
In February 2022, Health Canada released the report Health of Canadians in a Changing Climate – Advancing our Knowledge for Action, which provided an assessment of the risks of climate change to the health of Canadians and to the health care system, including a chapter on health equity.
The Department conducted an online survey; participants included parents/guardians of children aged 6 and under, pregnant individuals or those planning to become pregnant in the next six months, seniors, and newcomers to Canada, to determine their awareness and understanding of chemicals and pollutants, as well as their actual behaviours related to these risks at home. Research findings will be used to help develop messaging and resources.
Cannabis
Health Canada continued to integrate SGBA Plus into its population-based survey on cannabis. The Canadian Cannabis Survey added additional questions to better understand cannabis use and priority populations. Since the survey's launch, it has asked about gender, highest level of education, sexual orientation, and household income. In 2020, the Department added questions on race, ethnicity, and Indigenous identity, and adjusted these in 2021 to improve data quality.
Some key survey results from 2021 indicated a disparity in cannabis use among different demographic variables, including sex, gender, sexual orientation, age group and Indigenous identity. The results will allow the Department to better target its programs and public education to priority populations.
Health Canada also took steps to increase diversity in the regulated cannabis industry and to better understand barriers to participation. For example, it continued to offer a dedicated Indigenous Navigator Service, whose purpose is to help and support Indigenous-affiliated applicants throughout the federal commercial cannabis licensing process and encourage their participation in the industry.
The Department continued work to enable a diverse and competitive cannabis industry comprised of small and large businesses, and increase the participation of Indigenous, Black and other racialized communities by developing guidance and improving communication with industry stakeholders, supporting prospective applicants in entering the legal framework, and supporting licence holders in achieving and maintaining compliance with the Cannabis Act and its Regulations.
Controlled substances
Health Canada collected SGBA Plus data and information (such as sex, gender, age and region) from a variety of sources, including general and targeted population surveys (such as the Canadian Alcohol and Drugs Survey and the Canadian Student Drugs and Tobacco Survey), reports from supervised consumption sites, and recipients of SUAP funding. The Department developed methods to ensure sample sizes were adequate for meaningful SGBA Plus analysis.
Opioid overdose crisis
Health Canada used disaggregated data to define issues and inform policies and programs related to the opioid overdose crisis as well as to identify elevated harms in key demographics.
Further to previous studies in 2017 and 2019, the Department also conducted the Follow-up Survey and Qualitative Research on Opioid Awareness, Knowledge and Behaviours for Public Education to support the development of evidence-based public education materials around the overdose crisis and other substance use.
Chemicals management
Health Canada piloted new tools and training materials to strengthen and more systematically apply SGBA Plus considerations within its chemicals management activities, including risk assessment, risk management, engagement, outreach, as well as air and water quality.
Under the Pest Control Products Act, Health Canada continued to consider SGBA Plus metrics (including sex, gender, behaviour, age, occupation, social, and cultural factors) during the risk assessment process when regulating pesticides.
Environmental health
In February 2022, Health Canada released the report Health of Canadians in a Changing Climate – Advancing our Knowledge for Action, which provided an assessment of the risks of climate change to the health of Canadians and to the health care system, including a chapter on health equity.
The Department continued to refine its environmental health outreach materials to support inclusive outcomes for all, with an emphasis on inclusive images and text, and described video to challenge stereotypes. The findings of an online survey reaching out to parents/guardians of children aged 6 and under, pregnant individuals (or those planning to), seniors, and newcomers to Canada will be used to improve messaging and resources.
In 2021-22, the Department identified community organizations that delivered services to individuals of different races, ethnicities, religious affiliations, education levels, gender, sex, employment and age demographics for its Healthy Home Campaign train-the-trainer projects, to ensure messaging would reach underserved, at-risk populations. The selected organizations were able to use their knowledge of trends within the communities they service to create inclusive, dynamic and flexible programming specific to local needs.
Health Canada developed a new 3-year Healthy Home marketing strategy to encourage individuals to take action to protect themselves and their families from chemicals and pollutants in and around the home. In addition to homeowners and renters in general, the strategy focused on reaching vulnerable and at-risk populations.
Spending and human resources
Spending
Spending 2019-20 to 2024-25
The following graph presents planned (voted and statutory spending) over time.
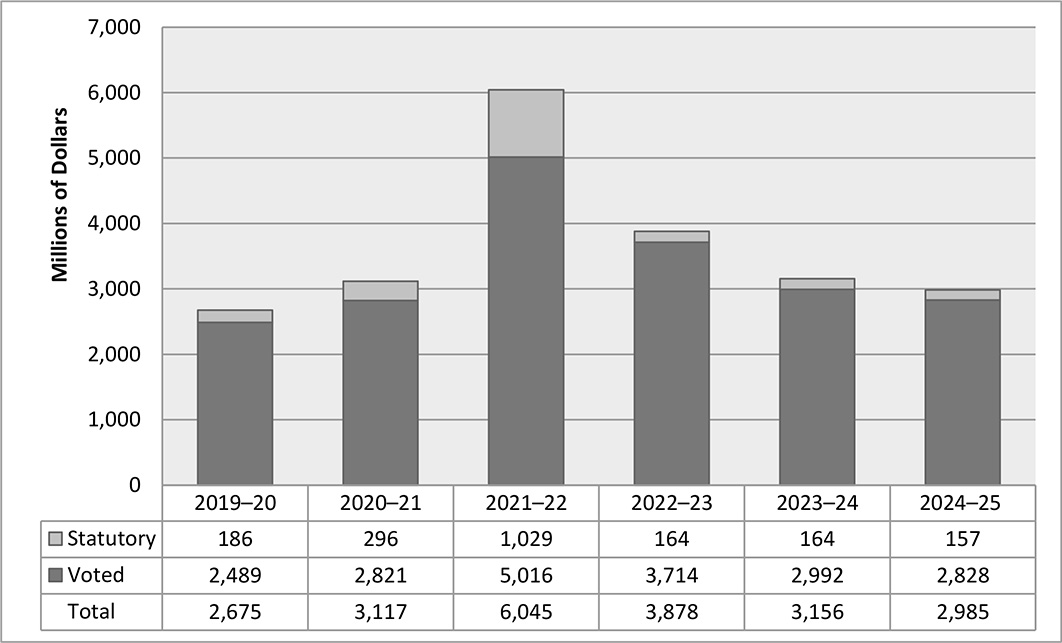
Text description
The figure illustrates Health Canada's spending trend from fiscal year 2019-20 to fiscal year 2024-25 where spending, in millions of dollars, is shown on the vertical axis and time period, in fiscal years, is shown on the horizontal axis. Health Canada's actual spending for fiscal year 2019-20: $2,675 million (Voted: $2,489 million, Statutory: $186 million); 2020-21: $3,117 million (Voted: $2,821 million, Statutory: $296 million); and 2021-22: $6,045 million (Voted: $5,016 million, Statutory: $1,029 million).
Health Canada's planned spending for fiscal year 2022-23: $3,878 million (Voted: $3,714 million, Statutory: $164 million); 2023-24: $3,156 million (Voted: $2,992 million, Statutory: $164 million); and 2024-25: $2,985 million (Voted: $2,828 million, Statutory: $157 million).
Budgetary performance summary for core responsibilities and internal services (dollars)
The "Budgetary performance summary for core responsibilities and internal services" table presents the budgetary financial resources allocated for Health Canada's core responsibilities and for internal services.
| Core responsibilities and internal services | 2021–22 main estimates |
2021–22 planned spending |
2022–23 planned spending |
2023–24 planned spending |
2021–22 total authorities available for use | 2021–22 actual spending (authorities used) | 2020–21 actual spending (authorities used) | 2019–20 actual spending (authorities used) |
|---|---|---|---|---|---|---|---|---|
| Core Responsibility 1: Health Care Systems | 2,456,807,897 | 2,456,807,897 | 2,851,114,525 | 2,257,744,222 | 7,906,983,369 | 4,744,300,568 | 1,987,223,947 | 1,601,069,150 |
| Core Responsibility 2: Health Protection and Promotion | 1,104,086,037 | 1,104,086,037 | 750,221,957 | 617,360,824 | 986,642,487 | 787,250,023 | 660,580,250 | 728,899,756 |
| Subtotal | 3,560,893,934 | 3,560,893,934 | 3,601,336,482 | 2,875,105,046 | 8,893,625,856 | 5,531,550,591 | 2,647,804,197 | 2,329,968,906 |
| Internal Services | 301,904,724 | 301,904,724 | 276,665,409 | 281,252,239 | 555,219,990 | 513,234,110 | 468,848,746 | 345,420,163 |
| Total | 3,862,798,658 | 3,862,798,658 | 3,878,001,891 | 3,156,357,285 | 9,448,845,846 | 6,044,784,701 | 3,116,652,943 | 2,675,389,069 |
Note: At the outset of the 2021-22 fiscal year, Health Canada's planned spending was $3,862.8 million. Additional in-year funding received for Health Canada's response to the COVID-19 pandemic, Treasury Board approved initiatives as well as the operating and capital budget carry forwards increased Health Canada's total authorities to $9,448.8 million. The additional funding received during 2021-22 relates mainly to the following: procurement of additional COVID-19 rapid test kits; investments in LTC; supporting emergency measures related to the pandemic; the Safe Restart Agreement for federal investments in testing, contact tracing and data management; drugs, medical devices and virtual care; continuing Canada's chemical management regime; regulatory and operational functions to support critical COVID-19 focused operations; as well as addressing the opioid overdose crisis and problematic substance use. The variance of $3.4 billion between total authorities and actual spending in 2021-22 is mainly the result of the reprofile of COVID-19 related funds from 2021-22 to 2022-23 in order to continue Health Canada's response to the pandemic, as well as Health Canada requesting both statutory and voted spending authorities allowing for flexibility for the procurement of critical and time-sensitive additional COVID-19 rapid tests. These expenses were charged to either the statutory or the voted authorities – not both. For any expenses charged to the statutory authorities, an equal amount was frozen in the voted appropriations. Fiscal year 2021-22 actual spending increased significantly compared to prior fiscal years due to Health Canada's response to the COVID-19 pandemic. |
||||||||
Human Resources
The "Human resources summary for core responsibilities and internal services" table presents the full-time equivalents (FTEs) allocated to each of Health Canada's core responsibilities and to internal services.
| Core Responsibilities and Internal Services | 2019–20 actual FTEs |
2020–21 actual FTEs |
2021–22 planned FTEs |
2021–22 actual FTEs |
2022–23 planned FTEs |
2023–24 planned FTEs |
|---|---|---|---|---|---|---|
| Core Responsibility 1: Health Care Systems | 215 | 247 | 276 | 428 | 285 | 279 |
| Core Responsibility 2: Health Protection and Promotion | 5,785 | 6,036 | 5,933 | 6,527 | 5,610 | 5,627 |
| Subtotal | 6,000 | 6,283 | 6,209 | 6,955 | 5,895 | 5,906 |
| Internal Services | 2,164 | 2,344 | 1,804 | 2,573 | 1,698 | 1,724 |
| Total | 8,164 | 8,627 | 8,013 | 9,528 | 7,593 | 7,630 |
Note: The variance in FTE utilization in 2021-22 is mainly due to the additional in-year resources received for continuing Canada's chemical management regime; regulatory and operational functions to support critical COVID-19 focused operations; addressing the opioid overdose crisis and problematic substance use; as well as ensuring the ongoing integrity of the Public Service Occupational Health Program. FTE utilization in 2021-22 increased significantly compared to prior fiscal years due to Health Canada's response to the COVID-19 pandemic. The decrease in planned FTEs in 2022-23 is mainly due to the expiry of budgetary authorities in 2021-22 for the federal framework to legalize and regulate cannabis; funding to support regulatory and operational critical COVID-19 focused functions; as well as the safe restart agreement for federal investments in testing, contact tracing, data management. The Department will have to request funding for these initiatives for future years. |
||||||
Expenditures by vote
For information on Health Canada's organizational voted and statutory expenditures, consult the Public Accounts of Canada 2020-21.
Government of Canada spending and activities
Information on the alignment of Health Canada's spending with the Government of Canada's spending and activities is available in GC InfoBase.
Financial statements and financial statements highlights
Financial statements
Health Canada's financial statements (unaudited) for the year ended March 31, 2022, are available on the departmental website.
Financial statements highlights
| Financial information | 2021–22 planned results |
2021–22 actual results |
2020–21 actual results |
Difference (2021–22 actual results minus 2021–22 planned results) |
Difference (2021–22 actual results minus 2020–21 actual results) |
|---|---|---|---|---|---|
| Total expenses | 4,201,583,642 | 5,937,735,922 | 3,450,839,105 | 1,736,152,280 | 2,486,896,817 |
| Total revenues | 222,924,854 | 407,154,364 | 305,848,970 | 184,229,510 | 101,305,394 |
| Net cost of operations before government funding and transfers | 3,978,658,788 | 5,530,581,558 | 3,144,990,135 | 1,551,922,770 | 2,385,591,423 |
The Department's total expenses in 2021-22 were $5,937.7 million. There was an increase of total expenses of $1,736.2 million when comparing actual results against planned results for 2021-22. This is primarily due to:
When comparing year-over-year actual expenditures, there was an increase of $2,486.9 million. The significant changes were:
The Department's total revenues were $407.2 million in 2021-22 representing an increase of $184.2 million from planned results and an increase of $101.3 million over the prior year actual revenues. The year-over-year variance is primarily a result of an increase in revenues from PHAC for additional services supporting COVID-19 initiatives, provided under the Shared Services Partnership agreement, annual increases to specific fee regimes, an increase in volume of regulatory reviews and evaluations for medical devices, drugs and vaccines available for sale and use due to COVID-19. |
|||||
The 2021–22 planned results information is provided in Health Canada's Future-Oriented Statement of Operations and Notes 2021–22.
| Financial information | 2021–22 | 2020–21 | Difference (2021–22 minus 2020–21) |
|---|---|---|---|
| Total net liabilities | 2,060,339,128 | 404,277,349 | 1,656,061,779 |
| Total net financial assets | 1,922,479,315 | 273,954,333 | 1,648,524,982 |
| Departmental net debt | 137,859,813 | 130,323,016 | 7,536,797 |
| Total non-financial assets | 581,374,300 | 142,590,871 | 438,783,429 |
| Departmental net financial position | 443,514,487 | 12,267,855 | 431,246,632 |
Total net liabilities were $2,060.3 million at the end of 2021-22, representing an increase of $1,656.1 million from the previous year. This variance is mainly due to timing of payments for transfer payment agreements approved during the latter part of the fiscal year, and amounts owing for the acquisition of rapid test kits where goods were received shortly before year end. The year-over-year increase in total net financial assets of $1,648.5 million is primarily a result of an increase in amounts due from the Consolidated Revenue Fund, which is reflective of the increase in accounts payable noted above. Total non-financial assets were $581.4 million at the end of 2021-22, representing an increase of $438.8 million from the previous year. This increase reflects the inventory of rapid test kits held at year end, as well as advance payments issued to secure the acquisition of rapid test kits. |
|||
Corporate information
Organizational profile
Appropriate Ministers: The Honourable Jean-Yves Duclos, P.C., M.P.
and The Honourable Dr. Carolyn Bennett, P.C., M.P.
Institutional Head: Dr. Stephen Lucas
Ministerial portfolio: Health
Enabling instrument[s]: Assisted Human Reproduction Act, Canada Health Act, Canada Consumer Product Safety Act, Cannabis Act, Controlled Drugs and Substances Act, Department of Health Act, Food and Drugs Act, Hazardous Materials Information Review Act, Hazardous Products Act, Pest Control Products Act, Radiation Emitting Devices Act, Tobacco and Vaping Products Act.
Year of incorporation / commencement: 1913
Raison d'être, mandate and role
"Raison d'être, mandate and role: who we are and what we do" is available on Health Canada's website.
For more information on the Department's organizational mandate letters commitments, see the mandate letters for the Minister of Health and Minister of Mental Health and Addictions and Associate Minister of Health.
Operating context
Information on the operating context is available on the Health Canada website.
Reporting framework
Health Canada's approved Departmental Results Framework and Program Inventory of record for 2021–22 are shown below.

Legend:
R: Result
I: Indicator
Text description
The figure illustrates Health Canada's approved Departmental Results Framework and Program Inventory for 2021-22.
The Departmental results Framework groups Health Canada's Core Responsibilities into two categories, all supported by Internal Services. These categories are (1) Health Care Systems and (2) Health Protection and Promotion, each of which are delivered through multiple programs in the Program Inventory. Each core responsibility has departmental results and several indicators associated with it.
Departmental Results Framework
Core Responsibility 1: Health Care Systems
- R 1: Canada has modern and sustainable health care systems
- I1: National health expenditure as a percentage of Gross Domestic Product
- I2: Real per capita health expenditure
- I3: Drug spending as a percentage of Gross Domestic Product
- I4: Percentage of family physicians using electronic medical records
- R 2: Canadians have access to appropriate and effective health services
- I5: Percentage of Canadians (aged 15+) with a mental disorder who have expressed that they have an unmet mental health care need
- I6: Percentage of Canadians (aged 15+) who have expressed that they have an unmet need for access to home care services
- I7: Percentage of Canada Health Act compliance issues addressed within 24 months of identification
- I8: Percentage of Canadians who did not fill a prescription for medicine because of the cost
Program Inventory under core responsibility one (from one - 15) as follow:
- Health Care Systems Analysis & Policy
- Access, Affordability, & Appropriate Use of Drugs & Medical Devices
- Home, Community & Palliative Care
- Mental Health
- Digital Health
- Health Information
- Canada Health Act
- Medical Assistance in Dying
- Cancer Control
- Patient Safety
- Blood Systems, Organs, Tissue & Transplantation
- Promoting Minority Official Languages in the Health Care Systems
- Brain Research
- Thalidomide
- The Territorial Health Investment Fund (THIF)
Core Responsibility 2: Health Protection and Promotion
- R 3: Canadians have access to safe, effective and quality health products
- I9: Percentage of new drug decisions issued within service standards
- I10: Percentage of Risk Management Plan reviews for new drug decisions completed within service standards
- I11: Percentage of drug companies deemed to be compliant with manufacturing requirements under the Food and Drugs Act and associated regulations
- R 4: Canadians are protected from unsafe consumer and commercial products and substances
- I12: Percentage of domestic consumer product recalls communicated to Canadians in a timely manner
- I13: Percentage of actions taken in a timely manner to protect the health of Canadians from substances found to be a risk to human health
- I14: Percentage of actions taken in a timely manner to protect the health of Canadians from pesticides found to be a risk to human health and the environment
- R 5: Canadians make healthy choices
- I15: Percentage of Canadians (aged 15+) who have used any tobacco product in the past 30 days
- I16: Percentage of Canadians (aged 15-24) who have used cannabis in the last 12 months
- I17: Percentage of Canadians who use dietary guidance provided by Health Canada
Program Inventory under core responsibility two (from 16-32) as follow:
- Pharmaceutical Drugs
- Biologics & Radiopharmaceutical Drugs
- Medical Devices
- Natural Health Products
- Food & Nutrition
- Air Quality
- Climate Change
- Water Quality
- Health Impacts of Chemicals
- Consumer Product Safety
- Workplace Hazardous Products
- Tobacco Control
- Controlled Substances
- Cannabis
- Radiation Protection
- Pesticides
- Specialized Health Services & Internationally Protected Persons Program
Internal Services
Supporting information on the program inventory
Financial, human resources and performance information for Health Canada's Program Inventory is available in GC InfoBase.
Supplementary information tables
The following supplementary information tables are available on Health Canada's website:
- Departmental Sustainable Development Strategy/Reporting on Green Procurement
- Details on transfer payment programs
- Gender-based analysis plus
- Response to parliamentary committees and external audits
- Horizontal initiatives
- Up-front multi-year funding
Federal tax expenditures
The tax system can be used to achieve public policy objectives through the application of special measures such as low tax rates, exemptions, deductions, deferrals and credits. The Department of Finance Canada publishes cost estimates and projections for these measures each year in the Report on Federal Tax Expenditures. This report also provides detailed background information on tax expenditures, including descriptions, objectives, historical information and references to related federal spending programs as well as evaluations and GBA Plus of tax expenditures.
Organizational contact information
Serena Francis
Assistant Deputy Minister / Chief Financial Officer
Health Canada
Assistant Deputy Minister Office
70 Colombine Driveway, Tunney's Pasture
Ottawa, Ontario K1A 0K9
Telephone: 613-948-6358
serena.francis@hc-sc.gc.ca
Appendix: definitions
- appropriation (crédit)
- Any authority of Parliament to pay money out of the Consolidated Revenue Fund.
- budgetary expenditures (dépenses budgétaires)
- Operating and capital expenditures; transfer payments to other levels of government, organizations or individuals; and payments to Crown corporations.
- core responsibility (responsabilité essentielle)
- An enduring function or role performed by a department. The intentions of the department with respect to a core responsibility are reflected in one or more related departmental results that the department seeks to contribute to or influence.
- Departmental Plan (plan ministériel)
- A report on the plans and expected performance of an appropriated department over a 3-year period. Departmental Plans are usually tabled in Parliament each spring.
- departmental priority (priorité)
- A plan or project that a department has chosen to focus and report on during the planning period. Priorities represent the things that are most important or what must be done first to support the achievement of the desired departmental results.
- departmental result (résultat ministériel)
- A consequence or outcome that a department seeks to achieve. A departmental result is often outside departments' immediate control, but it should be influenced by program-level outcomes.
- departmental result indicator (indicateur de résultat ministériel)
- A quantitative measure of progress on a departmental result.
- departmental results framework (cadre ministériel des résultats)
- A framework that connects the department's core responsibilities to its departmental results and departmental result indicators.
- Departmental Results Report (rapport sur les résultats ministériels)
- A report on a department's actual accomplishments against the plans, priorities and expected results set out in the corresponding Departmental Plan.
- experimentation (expérimentation)
- The conducting of activities that seek to first explore, then test and compare the effects and impacts of policies and interventions in order to inform evidence-based decision-making, and improve outcomes for Canadians, by learning what works, for whom and in what circumstances. Experimentation is related to, but distinct from innovation (the trying of new things), because it involves a rigorous comparison of results. For example, using a new website to communicate with Canadians can be an innovation; systematically testing the new website against existing outreach tools or an old website to see which one leads to more engagement, is experimentation.
- full-time equivalent (équivalent temps plein)
- A measure of the extent to which an employee represents a full person-year charge against a departmental budget. For a particular position, the full-time equivalent figure is the ratio of number of hours the person actually works divided by the standard number of hours set out in the person's collective agreement.
- gender-based analysis plus (GBA Plus) (analyse comparative entre les sexes plus [ACS Plus])
- An analytical tool used to support the development of responsive and inclusive policies, programs and other initiatives; and understand how factors such as sex, race, national and ethnic origin, Indigenous origin or identity, age, sexual orientation, socio-economic conditions, geography, culture and disability, impact experiences and outcomes, and can affect access to and experience of government programs.
- government-wide priorities (priorités pangouvernementales)
- For the purpose of the 2021–22 Departmental Results Report, government-wide priorities refers to those high-level themes outlining the government's agenda in the 2020 Speech from the Throne, namely: Protecting Canadians from COVID-19; Helping Canadians through the pandemic; Building back better – a resiliency agenda for the middle class; The Canada we're fighting for.
- horizontal initiative (initiative horizontale)
- An initiative where two or more federal organizations are given funding to pursue a shared outcome, often linked to a government priority.
- non-budgetary expenditures (dépenses non budgétaires)
- Net outlays and receipts related to loans, investments and advances, which change the composition of the financial assets of the Government of Canada.
- performance (rendement)
- What an organization did with its resources to achieve its results, how well those results compare to what the organization intended to achieve, and how well lessons learned have been identified.
- performance indicator (indicateur de rendement)
- A qualitative or quantitative means of measuring an output or outcome, with the intention of gauging the performance of an organization, program, policy or initiative respecting expected results.
- performance reporting (production de rapports sur le rendement)
- The process of communicating evidence-based performance information. Performance reporting supports decision making, accountability and transparency.
- plan (plan)
- The articulation of strategic choices, which provides information on how an organization intends to achieve its priorities and associated results. Generally, a plan will explain the logic behind the strategies chosen and tend to focus on actions that lead to the expected result.
- planned spending (dépenses prévues)
-
For Departmental Plans and Departmental Results Reports, planned spending refers to those amounts presented in Main Estimates.
A department is expected to be aware of the authorities that it has sought and received. The determination of planned spending is a departmental responsibility, and departments must be able to defend the expenditure and accrual numbers presented in their Departmental Plans and Departmental Results Reports.
- program (programme)
- Individual or groups of services, activities or combinations thereof that are managed together within the department and focus on a specific set of outputs, outcomes or service levels.
- program inventory (répertoire des programmes)
- Identifies all the department's programs and describes how resources are organized to contribute to the department's core responsibilities and results.
- result (résultat)
- A consequence attributed, in part, to an organization, policy, program or initiative. Results are not within the control of a single organization, policy, program or initiative; instead they are within the area of the organization's influence.
- statutory expenditures (dépenses législatives)
- Expenditures that Parliament has approved through legislation other than appropriation acts. The legislation sets out the purpose of the expenditures and the terms and conditions under which they may be made.
- target (cible)
- A measurable performance or success level that an organization, program or initiative plans to achieve within a specified time period. Targets can be either quantitative or qualitative.
- voted expenditures (dépenses votées)
- Expenditures that Parliament approves annually through an appropriation act. The vote wording becomes the governing conditions under which these expenditures may be made.
Page details
- Date modified:
