International Health Regulations - Joint External Evaluation of Canada Self-Assessment Report
April 20, 2018
Public Health Agency of Canada
Foreword
The world we live in today is characterised by the movement of information, products and people at unprecedented levels. While these exchanges have innumerable benefits, they also present new types of risks, particularly to health. It is imperative that risks be mitigated and managed by a strong health system with appropriate ties to the authorities responsible for emergency management, borders, and national security.
All 196 States Parties to the International Health Regulations (2005), including Canada, recognize that protecting health security requires all countries to establish public health core capacities to prevent, detect, and respond to significant events with health consequences. Over the last 20 years, infectious disease outbreaks including SARS, H1N1, Ebola in West Africa, and Zika, have amplified the need for a global approach to health security. Canada's own experience and participation in international response to public health emergencies has allowed us to see clearly the extent of the consequences public health emergencies can have on a society, and the pressing need to ensure measures are in place to prevent and mitigate these kinds of events. The Joint External Evaluation will provide Canada with an evidence-based set of recommendations to build national resilience and contribute to international health security.
Municipal, provincial, territorial, and federal levels of government have established innovative programs and capacities to protect the health of their residents and respond quickly to emerging risks. Completing an external review of Canadian core public health capacities is an opportunity to highlight our country's successes, but also to reflect on persisting challenges and imagine new ways of improving our systems.
We are proud to acknowledge the body of evidence gathered here through extensive collaboration with jurisdictional partners in health and other sectors. This report describes Canada's progress on 48 indicators across 19 technical areas. It provides a unique overview of Canada's health system and current state of public health preparedness. Our hope is that it will inspire future research and encourage innovation at local, provincial, regional and national levels.
Siddika Mithani, PhD
President, Public Health Agency of Canada
Dr. Theresa Tam
Chief Public Health Officer of Canada
Canada's health System
Canada is the second largest country in the world by total area covering 9.9 million square kilometres. Canada is home to 36.7 million people, more than 80,000 known wildlife species, and almost 200 million livestock. Although it has one of the world's lowest human population densities (4 people per km2), most Canadians (76%) live in urban centres along the border with the United States, with more than a third (36%) of the population living in the country's three largest cities: Toronto, Montreal or Vancouver.
Canada is a constitutional monarchy with multiple levels of government, including federal, provincial or territorial, and indigenous self-governments. Each level of government has its own areas of responsibility, but there are also areas where governments share responsibility. The federal government deals with areas of law listed in the Constitution Act, 1867 and that generally affect the whole country, such as national defence, foreign affairs, and Aboriginal lands and rights. Through "equalization payments" the federal government plays a role in addressing fiscal disparities between provinces and in ensuring that standards of health, education and welfare are the same for every Canadian.
Provincial and territorial governments have the power to make laws that affect their province or territory directly and to manage their own public lands. They are also responsible for health care delivery and education. Municipal governments run cities, towns or districts, including managing community water systems, local public land, and emergency first responders (police, fire protection, ambulances).
Indigenous self-government is part of Canada's evolving system of cooperative federalism and distinct orders of government. First Nations communities in Canada have a separate autonomous governance structure of elected band councils with responsibilities and authorities similar to those of municipal governments and with increasing powers over health in their communities.
Agriculture, immigration and health are some of the areas where the federal government and provinces and territories share responsibility.
Disease in Canada
In general, Canadians experience good health on a number of measures-almost 90% of Canadians report having good to excellent health, 92% say their lives are satisfying or very satisfying, and 70% of Canadians report having very good or excellent mental health. Canada's average life expectancy of 82 years ranks it among the healthiest nations in the world.
Canada has made great advances in preventing and controlling infectious diseases, through widespread improvements in hygiene and sanitation, water treatment systems, food safety measures, mass immunization programs, research into and development of new drugs, and education campaigns around safe sex, handwashing and safe food preparation. Canada also has better surveillance systems in place, providing a clearer picture of immunization rates and the distribution of diseases. Despite these advances, Canadians are still getting sick from infectious diseases and some of this sickness is long-term and treatment resistant, creating situations of vulnerability. In addition, some Canadians are not as healthy as others or are at a higher risk for poor health outcomes. Indigenous and low income households in Canada, for example, still live with higher rates of inadequate housing and food insecurity, compared to other Canadians. As well, Canada's geography, population distribution, and cultural differences create unique challenges to the delivery of health services in the country's northern, remote and rural communities.
While chronic diseases like diabetes, cancer, and cardiovascular disease are the leading causes of morbidity and mortality in Canada, infectious diseases continue to impose a significant burden on populations and health systems. Infectious disease priorities in Canada include, rates of antimicrobial resistance (AMR); vaccine coverage and the re-emergence of childhood vaccine-preventable diseases, such as measles and whooping cough; increasing rates of sexually transmitted and blood-borne infections; vector-borne diseases emerging as a result of climate change, such as Lyme disease; increasing rates of salmonella; and the disproportionately high rates of tuberculosis among foreign-born, First Nations and Inuit populations in Canada.
Although overall Canada's AMR rates are relatively low, there are upward trends in the rate of methicillin-resistant Staphylococcus aureus (MRSA) blood stream infection (BSI) in pediatric hospitals; in the rate of vancomycin-resistant Enterococcus (VRE) BSI in adult hospitals; and in the rates of drug-resistant gonorrhea in Canada.
The incidence of vaccine-preventable diseases in Canada is low. However, the viruses and bacteria that cause these diseases circulate within Canada and around the world and can still potentially cause outbreaks in under- and un-immunized groups. Since 2005 Canada has had a number of imported cases of measles resulting in spread and outbreaks within Canada. These importation events underline the ongoing risk of resurgence and the importance of achieving and maintaining Canada's vaccination coverage goals. Canada's public health and surveillance efforts have continued to maintain elimination of endemic measles in Canada. Raising immunization rates for measles, diphtheria, pertussis and tetanus in Canada-which currently are below national coverage goals of 97% by age two years-will further guard against the spread of these diseases following importation by travellers returning from endemic countries.
Government investments have contributed to the prevention and control of some sexually transmitted and blood borne infections. However, new HIV and hepatitis C infections continue to occur among certain populations and reported rates of chlamydia, gonorrhea and syphilis in Canada have been steadily rising since the late 1990s.
Climate change has been implicated in rising rates of vector‐borne communicable diseases in Canada. West Nile Virus appeared in Canada for the first time in 2002 and the incidence of Lyme disease has been increasing as higher temperatures allow mosquitoes and ticks to spread within Canada.
Serious outbreaks of food-borne diseases are rare in Canada. However, food-borne bacteria, parasites and viruses still cause illnesses in Canada. Every year, about 4 million (1 in 8) Canadians are affected by a food-borne illness. Over the last three years salmonella rates in Canada have increased mainly attributed to a rise in the incidence of salmonella enteriditis.
Canada has one of the lowest incidence rates of tuberculosis in the world at 4.4 cases per 100,000 population. Although the overall incidence of tuberculosis in Canada has steadily decreased over the last 30 years, tuberculosis continues to disproportionately affect First Nations and Inuit populations, with incidence rates of 20.4 per 100,000 and 198.3 per 100,000, respectively.
Diseases and conditions linked to unhealthy living (diabetes, obesity and mood disorders) have been increasing in Canada. Over a relatively short period of time, for example, the proportion of Canadians living with diabetes almost doubled from 6% in 2000 to 10% in 2011. This is a concern because type 2 diabetes is linked to higher proportions of people with an unhealthy diet, low physical activity and higher rates of obesity, all of which are linked to a variety of other diseases and conditions, making them proxies for overall health. Moreover, the gap between the highest and lowest income groups is widening. In 2014 Canadians in the lowest income group were twice as likely to report living with cardiovascular disease as those in the highest income group.
As a major exporter of live animals and animal products, and also a significant importer of animal products, and some live animals, Canada adopts a very rigorous approach to identifying and mitigating possible risks and has strict border controls in place. Despite the low levels of accepted risk, Canada has faced a number of major disease challenges in recent years including bovine spongiform encephalopathy, avian influenza and bovine tuberculosis. These outbreaks of foreign animal diseases have been effectively managed and the diseases either eliminated or are in the process of being controlled. Canada has also implemented a number of effective disease control and eradication programmes, including programs against tuberculosis and brucellosis.
Key features of Canada's health care system
Canada's core publicly funded health care system, known as Medicare, provides universal coverage for medically necessary hospital and physician services-patients do not pay user fees. While there are government programs that provide access to non-Medicare services for certain groups, such as children, seniors, and people with low incomes, the majority of working Canadians have private insurance plans to pay for services not covered by Medicare, such as prescription drugs outside of hospitals, vision care, dental care and physiotherapy. Quebec is the only province with a universal prescription drug plan. If neither public nor private insurance covers the full cost of a service, patients must pay out-of-pocket. While the Canadian health care system is mostly publicly funded through taxes, health care services are provided by a mix of public and private organizations and self-employed professionals.
The Canada Health Act sets out the criteria and conditions that provincial and territorial health care insurance plans must meet in order to receive the full federal cash transfer (Canada Health Transfer) to which they are entitled under the act. The five principles enshrined in the act are:
- Public administration: public health care insurance plans must be administered on a non-profit basis by a public authority
- Comprehensive: plans must cover all medically necessary hospital and physician services
- Universal: all eligible residents must be entitled to coverage on uniform terms and conditions.
- Portable: Coverage must be maintained when the insured person travels within Canada or abroad, within prescribed limits.
- Accessible: Reasonable access to insured health services must be maintained and unimpeded by financial or other barriers.
The act also discourages extra-billing and user charges for insured health services through mandatory dollar-for-dollar deductions from federal transfers.
Role of governments
Health care in Canada is primarily a provincial and territorial responsibility. Provinces and territories manage and deliver health care services in their jurisdictions (accounting for about 65% of Canada's total health expenditures). The federal government, through the Canada Health Act and other legislation, also plays an important role in matters that affect the health of Canadians, such as funding, regulating food and drugs, and setting national standards for health care.
Provinces fund and administer health insurance plans and other health care programs and they determine the organization and governance of their own health care systems. They regulate health care facilities and professionals, as well as private insurance; they manage capital investments; and they negotiate purchasing and pricing for their drug plans.
In provinces, regional health authorities plan, fund and deliver (within a defined geographical area) health care services, such as hospital care, rehabilitation and home care. However, regional health authorities are not responsible for physician services and drug plans, which remain the responsibility of provincial and territorial governments.
The planning and delivery of public health services in Canada is mostly done at the local or regional level through health departments of regional health authorities or districts or through health units and municipal health departments. These organizations have their own governance structures and their activities are governed by a provincial or territorial public health act (or equivalent) and its regulations, as well as by specific provincial or territorial legislation, policy, directives and conditions of funding--all of which vary from province to province. There is also considerable variation among public health units, which can serve populations from 600 to 2.4 million people with catchment areas from four to 800,000 square kilometers.
In addition, each province and territory has a chief medical officer of health (or equivalent) whose reporting relationship also varies considerably across the country as each province or territory tries to balance the independence of the CMOH as a health advocate with the need to integrate the portfolio into ministries of Health.
The federal government sets and administers national standards for Canada's health care system and funds provincial and territorial health care services through the Canada Health Transfer, an annual cash transfer to provinces and territories amounting to $37 billion in 2017-18 (about 23% of the total provincial and territorial health care expenditure). The federal government also regulates products, such as food, drugs, and pesticides, as well as medical and radiation-emitting devices; and it delivers or funds health care services to specific groups, including First Nations living on reserve, members of the Canadian Armed Forces, veterans, refugee claimants, and federal inmates. Indigenous Services Canada has a mandate to provide certain public health services to First Nations communities on reserve. Many Indigenous self-government agreements include the responsibility to deliver health care and public health services to their population.
All levels of government share responsibility for health care funding, health research and health promotion and protection, including emergency preparedness and response activities.
Funding
Canada spent about $242 billion on health in 2017. Although the system is predominantly publicly financed (70%), private financing (30%) plays an important role: private health insurance for services not covered by Medicare accounts for about 12% of Canada's health spending, while out-of-pocket payments by individuals for health services accounts for another 15%. Donations and other non-patient revenue streams make up the remaining 3% of private financing.
Besides the Canada Health Transfer to the provinces and territories, the federal government spent about $8 billion in 2017 (3% of total health spending) on direct health expenditures. Revenues for the publicly funded portion of health care expenditures come from federal, provincial and territorial tax revenues.
Health and medical research
Health research advances our understanding of the factors that influence health and plays an important role not only in improving health outcomes for Canadians but also in contributing to Canada's overall social and economic prosperity. Health research in Canada is supported by the federal and provincial governments, non-government organizations and industry. Most health research in Canada is conducted by the higher education sector (in association with research hospitals), industry and non-governmental organizations and some is conducted in the federal government's own facilities.
The Canadian Institutes of Health Research (CIHR) is Canada's major federal funder of health research. CIHR invests approximately $1 billion each year to support both investigator-driven (72%) and priority-driven (28%) health research in all four pillars: biomedical, clinical, health systems services, and population health. Chosen through a peer review process that ensures quality and fairness, top investigator-driven research proposals are funded through a variety of programs. Priority-driven research initiatives are created by the Government of Canada to investigate pressing health issues that are of strategic importance to the country.
CIHR's 13 institutes align their individual strategic plans with the overarching direction and goals of Canada's Health Research Roadmap II. Institutes work with stakeholders across disciplines, professions, sectors and geographic borders to identify health and health system needs and capture emerging national and international scientific opportunities.
Other federal funders of health research in Canada include the Natural Sciences and Engineering Research Council, Health Canada, Canadian Institute for Health Information, Genome Canada and the Canadian Foundation for Innovation.
Provincial research funding agencies also contribute to medical research and training in Canada through organizations such as Alberta Innovates, Fonds de recherche santé du Québec, Manitoba Health Research Council, Newfoundland and Labrador Centre for Applied Health Research, and British Columbia's Michael Smith Foundation for Health Research.
Self-assessment process
Canada announced at the North American Leaders' Summit in June 2016 its commitment to undergo a Joint External Evaluation (JEE) in June 2018. Project planning got underway in the fall of 2016, about 18 months before the planned site visit of the external evaluation team, and was achieved in four phases:
- Planning
- Stakeholder engagement
- Self-assessment
- External evaluation (site visits)
Led by the Public Health Agency of Canada (PHAC), the project involved 10 federal government departments and representatives from 13 provinces and territories.
A small project team was established within PHAC's Centre for Emergency Preparedness and Response. The project team worked with technical experts, policy analysts and senior managers within the Agency to design and hold stakeholder consultations, coordinate the gathering and validation of evidence for all 48 indicators, draft and produce Canada's self-assessment report, and plan and coordinate the external evaluation team site visit. (Table 1: Project Plan for Canada's Joint External Evaluation, June 2016 to June 2018)
The team identified technical leads-experts in each of the various technical areas-from PHAC, Health Canada, and the Canadian Food Inspection Agency to act as liaisons between the project team and program areas, to help gather data for the indicators, and to draft the self-assessment report's 19 technical chapters. Technical leads consulted with other experts within relevant federal program areas and with provincial and territorial colleagues, including a two-day meeting in November 2017 to discuss the evidence and to propose scores for each indicator.
| Phase | Activities |
|---|---|
| 01 Planning November 2016 to March 2017 |
Project team:
|
| 02 Stakeholder engagement January 2017 to March 2017 |
Project team:
|
| 03 Self-Assessment March 2017to December 2017 |
Project team:
Technical leads:
|
| 04 External Evaluation January 2018 to June 2018 |
Project team:
|
Self-assessment results
Indicator scores
Canada's Self-Assessment Report is an aggregate national assessment that was developed in consultation with federal, provincial and territorial governments. The indicator scores presented in table 2 below are the outcome of a two-day consultation with federal, provincial and territorial officials. The evidence in the technical chapters of this report and the proposed scores (on a scale of 1 "no capacity" to 5 "sustainable capacity") have been reviewed, validated and agreed upon by a wide range of government partners and technical experts.
| N/A | Indicator number | Description | Score |
|---|---|---|---|
| P1 Legislation | P1.1 | Legislation, laws, regulations, administrative requirements, policies or other government instruments are sufficient for implementation of IHR | 5 |
| P1.2 | The state can demonstrate that it has adjusted and aligned its domestic legislation, policies and administrative arrangements to enable compliance with the IHR | 4 | |
| P2 IHR Collaboration | P2.1 | A functional mechanism is established for coordination and integration of relevant sectors in the implementation of the IHR | 4 |
| P3 Antimicrobial resistance | P3.1 | Antimicrobial resistance detection | 3 |
| P3.2 | Surveillance of infections caused by antimicrobial resistant pathogens | 3 | |
| P3.3 | Healthcare associated infection prevention and control programs | 4 | |
| P3.4 | Antimicrobial stewardship activities | 3 | |
| P4 Zoonoses | P4.1 | Surveillance systems in place for priority zoonotic diseases/pathogens | 4 |
| P4.2 | Veterinary or animal health workforce | 4 | |
| P4.3 | Mechanisms for responding to infectious zoonoses and potential zoonoses are established and functional | 4 | |
| P5 Food safety | P5.1 | Mechanisms are established and functioning for detecting and responding to food borne disease and food contamination | 5 |
| P6 Biosafety and biosecurity | P6.1 | Whole-of-government biosafety and biosecurity system is in place for human, animal, and agriculture facilities | 5 |
| P6.2 | Biosafety and biosecurity training and practices | 4 | |
| P7 Immunization | P7.1 | Vaccine coverage (measles) as part of national program | 3 |
| P7.2 | National vaccine access and delivery | 5 | |
| D1 National laboratory system | D1.1 | Laboratory testing for detection of priority diseases | 5 |
| D1.2 | Specimen referral and transport system | 5 | |
| D1.3 | Effective modern point of care and laboratory based diagnostics | 5 | |
| D1.4 | Laboratory quality system | 4 | |
| D2 Real time surveillance | D2.1 | Indicator and event based surveillance systems | 5 |
| D2.2 | Interoperable, interconnected, electronic real-time reporting system | 4 | |
| D2.3 | Analysis of surveillance data | 5 | |
| D2.4 | Syndromic surveillance systems | 5 | |
| D3 Reporting | D3.1 | System for efficient reporting to WHO, FAO and OIE | 5 |
| D3.2 | Reporting network and protocols in country | 5 | |
| D4 Workforce development | D4.1 | Human resources are available to. Implement IHR core capacity requirements | 5 |
| D4.2 | Applied epidemiology training program in place | 5 | |
| D4.3 | Workforce strategy | 4 | |
| R1 Preparedness | R1.1 | Multi-hazard national public health emergency preparedness and response plan is developed and implemented | 5 |
| R1.2 | Priority public health risks and resources are mapped and utilized | 3 | |
| R2 Emergency response operations | R2.1 | Capacity to activate emergency response operations | 5 |
| R2.2 | Emergency operations centre operating procedures and plans | 5 | |
| R2.3 | Emergency operations program | 4 | |
| R2.4 | Case management procedures are implemented for IHR relevant hazards | 5 | |
| R3 Linking public health and security authorities | R3.1 | Public health and security authorities (law enforcement, border control, customs) are linked during a suspect or confirmed biological event | 4 |
| R4 Medical countermeasures | R4.1 | System is in place for sending and receiving medical countermeasures during a public health emergency | 5 |
| R4.2 | System is in place for sending and receiving health personnel during a public health emergency | 5 | |
| R5 Risk communications | R5.1 | Risk communication systems | 4 |
| R5.2 | Internal and Partner Communication and Coordination | 5 | |
| R5.3 | Public Communication | 4 | |
| R5.4 | Communication engagement with affected communities | 4 | |
| R5.5 | Dynamic listening and rumour management | 3 | |
| Points of entry | POE 1 | Routine capacities are established at points of entry | 5 |
| POE 2 | Effective public health response at points of entry | 5 | |
| Radiation emergencies | RE 1 | Mechanisms are established and functioning for detecting and responding to radiological and nuclear emergencies | 4 |
| RE 2 | Enabling environment is in place for management of Radiation Emergencies | 5 | |
| Chemical events | CE 1 | Mechanisms are established and functioning for detecting and responding to chemical events or emergencies | 4 |
| CE 2 | Enabling environment is in place for management of chemical events | 4 |
P1: National legislation, policy and finance
Joint external evaluation target: States Parties should have an adequate legal framework to support and enable the implementation of all of their obligations and rights to comply with and implement the IHR (2005). In some States Parties, implementation of the IHR (2005) may require new or modified legislation. Even where new or revised legislation may not be specifically required under the State Party's legal system, States may still choose to revise some legislation, regulations or other instruments in order to facilitate their implementation and maintenance in a more efficient, effective or beneficial manner. States Parties should ensure provision of adequate funding for IHR implementation through the national budget or another mechanism.
Level of capability in Canada
Canada implements the International Health Regulations (IHR) under existing legislation, regulations, policies and agreements in place at both the federal, and the provincial and territorial levels. An internal review conducted in 2010 found that the legislative and non-legislative measures taken by federal, provincial and territorial governments were sufficient to support implementation of the IHR in Canada.
As a federated state, Canada requires federal, provincial and territorial cooperation to implement the IHR. Provinces and territories have primary responsibility for health, public health and emergency response in their jurisdictions. Accordingly, they have their own legislation and regulations for governing these activities.
The federal government shares responsibility for public health with provinces and territories and delivers health care to specific populations (First Nations on reserve, Inuit, federal inmates and Canadian Armed Forces personnel). It also has responsibility and authority in sectors that affect public health. Examples of the core federal public health legislation and regulations are:
- Department of Health Act
- Public Health Agency of Canada Act
- Quarantine Act
- Human Pathogens and Toxins Act and Human Pathogens and Toxins Regulations
Federal legislation also governs sectors that affect public health, such as nuclear safety, radiation protection, food safety, and animal health.
National (federal-provincial-territorial) collaborating mechanisms, agreements, policies and plans are in place that clarify roles, help align legal and policy frameworks across jurisdictions, and ensure effective cooperation in emergencies. In particular, the Pan-Canadian Public Health Network plays a unique role in public health in Canada. It provides a national governance structure to support evidence-based decision making, information sharing and dissemination, and coordination and collaboration across jurisdictions. The Network has led to many important national agreements and plans, such as the Multi-lateral Information Sharing Agreement, the Federal, Provincial and Territorial Public Health Response Plan for Biological Events, and the Canadian Pandemic Influenza Plan for the Health Sector.
As well, Canada has a memorandum of understanding on the provision of mutual aid in relation to health resources during an emergency affecting the health of the public. The Operational Framework for Mutual Aid Requests for Health Care Professionals (OFMAR) puts the principles of the MOU into practice. This mutual aid agreement serves as an important means to ensure that all jurisdictions have the resources they need to respond to a public health emergency, including those that may not have the capacity to respond to a complex emergency.
Following the SARS outbreak in 2003, Canada strengthened its federal public health capacity by creating the Public Health Agency of Canada (the Agency) in 2004. Part of Canada's Health Portfolio, the Agency supports the Minister of Health in exercising the powers, functions and duties related to public health, which can be found in legislation such as the Department of Health Act. They can also result from programs approved by Cabinet and funded by Parliament through appropriations submitted for approval by the President of Treasury Board.
The Agency is the IHR National Focal Point for Canada. It facilitates coordination and collaboration of IHR implementation with provinces and territories and across the federal government. As an Agency program, the National Focal Point receives stable funding through the federal government's annual budgeting process. Canadian government organizations at all levels implement the IHR as part of their core mandate.
Canada is also an active participant in cross-border plans, agreements and networks (Global Health Security Initiative, North American Plan for Animal and Pandemic Influenza) that further, enable or strengthen national and international IHR compliance.
Government programs that deliver on IHR requirements are funded through regular annual budgeting processes. The federal government publishes online annual departmental reports on plans and priorities which must show budget allocations to support programs. Governments in Canada also make special funds available quickly to support emergency response activities.
Indicators
P1.1 Legislation, laws, regulations, administrative requirements, policies or other government instruments in place are sufficient for implementation of IHR
Canadian laws and regulations governing public health surveillance and response
Canada has an extensive range of legislation, regulations, policies and other instruments in place for governing public health surveillance, preparedness and response, and to support its compliance with the IHR and its ongoing strengthening of implementation.
Public health in Canada is a responsibility shared among federal, provincial, territorial and local governments. Provinces and territories have their own legislation governing emergency response. They also have public health legislation that establishes authority for public health surveillance and requires local public health staff to report notifiable diseases to public health officials. Provinces and territories, because they are responsible for delivering health services, are important partners and contributors of surveillance information in Canada.
The federal mandate to carry out surveillance is derived from the powers and obligations conferred on the Government of Canada by a number of acts, including the Department of Health Act and the Public Health Agency of Canada Act. The Department of Health Act gives the Minister of Health a broad mandate to protect Canadians against health risks and the spread of disease.
The Minister's duties, functions and powers include investigation and research into public health, including the monitoring of diseases and, subject to the Statistics Act, "the collection, analysis, interpretation and publication and distribution of information relating to public health."
The Public Health Agency of Canada was established to assist federal efforts to identify and reduce public health risk factors and to support national readiness for public health threats, including responding to a public health emergency. The Public Health Agency of Canada Act outlines the measures that the Agency can take in public health, including health surveillance and public health emergency preparedness and response.
The Public Health Agency of Canada Act mandates the Agency, in collaboration with its partners, "to contribute to federal efforts to identify and reduce public health risk factors and to support national readiness for public health threats." The act recognizes that public health surveillance is one of the public health measures that the Government of Canada undertakes through various programs and activities carried out by the Agency.
The Agency is responsible for assisting the Minister of Health in exercising or performing her functions relative to public health. Other departments within the Health Portfolio are Health Canada, the Canadian Food Inspection Agency, the Canadian Institutes for Health Research and the Patented Medicine Prices Review Board.
The Department of Health Act and the Public Health Agency of Canada Act do not expressly deal with the collection of personal information. However, under section 4 of the Privacy Act, the Agency can collect personal information for the purpose of carrying out programs and activities to assist the Minister in exercising her powers, duties and functions relative to public health, if the collection relates to that program or activity. Provinces and territories comply with the IHR obligation to disclose information for the purposes of managing a public health risk, in accordance with relevant domestic laws.
Other key federal legislation and regulations that enable Canada to meet its IHR obligations include:
- Quarantine Act: authorizes the measures that can be taken at points of entry or departure to control the spread of diseases that pose a significant risk to public health. This includes measures taken with respect to conveyances and their cargo.
- Potable Water on Board Trains, Vessels, Aircraft and Buses Regulations: governs the safety of water for drinking, hand washing, and preparation of food on certain conveyances.
- Human Pathogens and Toxins Act and Human Pathogens and Toxins Regulations: establishes a safety and security regime to protect the health and safety of the public against risks from human pathogens and toxins. The act takes a risk-based approach-identifying three risk groups-and covers obligations and prohibitions, licensing, standards, guidelines and enforcement.
- Food and Drugs Act and Food and Drug Regulations: establishes standards for the safety and nutritional quality of all foods sold in Canada. The act covers food content, as well as its manufacture, preparation, preservation, packaging and storing. It also authorizes enforcement measures, from inspections to seizures and destruction.
- Emergency Management Act: authorizes the Minister of Public Safety to coordinate emergency management activities with government institutions and in cooperation with the provinces and territories. This includes monitoring potential, imminent and actual emergencies and advising other ministers. The act also directs all federal ministers to identify risks in their area of responsibility and prepare emergency response plans for those risks.
- Health of Animals Act and the Health of Animals Regulations: protect animals and animal health, providing for the control of diseases and toxic substances that may affect land and water animals or that may be transmitted by animals to people. This includes segregation and inspection of animals, the importation of animal by-products, quarantine of imported animals, eradication of diseases, regulation of veterinary biologics, as well as permits and licencing and animal identification.
- Canadian Environmental Protection Act: aims to protect the environment and human health from pollution and toxic substances. The act covers pollution prevention through, among others, regulation of vehicle emissions and timelines for the management of toxic substances.
- Nuclear Safety and Control Act: establishes the Canadian Nuclear Safety Commission and authorizes it to regulate the development and use of nuclear energy and the production, possession and use of nuclear substances to limit the associated risks to national security, the health and safety of people and the environment.
- Nuclear Terrorism Act: strengthens authority to respond to nuclear terrorism threats and allows Canada to fulfill international commitments relative to nuclear security.
- Radiation Emitting Devices Act and Radiation Emitting Devices Regulations: authorizes Health Canada to set and enforce standards for the sale and use of these devices, including inspections, testing, and the provision of safety guidance.
- Transportation of Dangerous Goods Act: promotes public safety in the transportation of dangerous goods and covers, among other things, safety and security requirements, emergency response plans, containment and shipping rules and standards, inspection and monitoring, and certification.
- Hazardous Products Act: requires suppliers of hazardous products to communicate those hazards through product labels and safety data sheets as a condition of sale and importation.
- Privacy Act: protects the privacy of individuals with respect to personal information held by government institutions and covers the collection, use, disclosure, retention and disposal of that information in the administration of programs.
- Immigration and Refugee Protection Act has provisions related to the protection of public health (s. 38) and longstanding program structures for implementing these provisions, through health admissibility screening in the immigration system.
Policies and other government instruments
Because of the differences in legislation between the federal and provincial and territorial levels, Canada has mechanisms, agreements and plans in place (for example, the Health Portfolio Emergency Response Plan, the Canadian Pandemic Influenza Preparedness Planning Guidance for the Health Sector, and the Strategic Emergency Management Plan) that enable national coordination, particularly during public health emergencies that require federal involvement.
The Pan-Canadian Public Health Network is a national body that strengthens and enhances Canada's public health capacity and enables federal, provincial and territorial governments to work together on the day-to-day business of public health and to anticipate, prepare for and respond to public health events and threats.
The Network includes the Public Health Network Council, the Council of Chief Medical Officers of Health and three steering committees: Healthy Peoples and Communities; Public Health Infrastructure; and Communicable and Infectious Diseases. The Network was designed to support public health within a federated system where each level of government has its own area of responsibility. The Network's guiding principles are:
- Respect for the authority of each government to manage operations within their own domain
- Embrace differences in how governments exercise their public health responsibilities, set priorities and manage infrastructure
- Recognize that there is no "one size fits all" approach to public health
- Collaborate with non-governmental organizations
The Network's newly developed Blueprint for a Federated System for Public Health Surveillance in Canada (Blueprint), is a framework and action plan to formalize key elements of public health surveillance in Canada, such as governance, standards, ethics, information sharing, and performance measurement.
Canada's Multi-Lateral Information Sharing Agreement came into force in 2014. It is a legal agreement that establishes standards and deals with the sharing, use, disclosure and protection of public health information for infectious disease surveillance and public health emergency response. Until all technical annexes to the Agreement are completed, parts of an earlier federal, provincial and territorial Memorandum of Understanding on the Sharing of Information during a Public Health Emergency are still in effect.
In addition, the IHR National Focal Point for Canada-a funded program within the Agency-has joined with other federal departments and agencies to put in place policy and administrative arrangements to implement the IHR. Among these is a memorandum of understanding between the Agency and the Department of National Defence allowing the Department of National Defence to inspect and issue ship sanitation control certificates and ship sanitation control exemption certificates for Canadian Armed Forces vessels.
The IHR National Focal Point did a Privacy Impact Assessment in 2016 to ensure its compliance with Canada's Privacy Act in its collection, retention and distribution of information as part of the requirements under the IHR. The Assessment did not identify any high-level risks.
The IHR National Focal Point is also coordinating the development of guidance on how international case and contact notices (that might be shared with other States Parties National Focal Points) are managed from receipt to retention and distribution, ensuring compliance with relevant Canadian laws and policies.
The Federal Nuclear Emergency Plan describes the Government of Canada's preparation and response arrangements for managing the radiological health consequences of a nuclear emergency. The Plan has provincial annexes for jurisdictions having nuclear power plants or ports visited by nuclear powered vessels. These annexes establish the link between federal and provincial nuclear emergency response organizations and capabilities.
Canada is a signatory to the Convention on Early Notification of a Nuclear Accident and has arrangements in place for timely notification of a nuclear accident to the International Atomic Energy Agency, and for coordinating with the IHR National Focal Point to report to the World Health Organization (WHO). (See also section RE: Radiation emergencies.)
Examples of cross-border agreements supporting health security
Canada is an active partner in several formal and informal cross-border agreements and networks that support the IHR, health security, and collaboration at the global, regional and sub-regional levels. These include the Global Health Security Initiative, the Global Health Security Agenda, and the North American Plan for Animal and Pandemic Influenza (NAPAPI).
NAPAPI supports IHR implementation. It is a framework for comprehensive health security across all relevant sectors in the North American region. Its function is to protect against, control, and provide a public health response to animal and pandemic influenza in North America while avoiding unnecessary interference with international travel and trade.
NAPAPI complements national emergency management plans in the United States, Canada and Mexico. It builds on the principles of the International Partnership on Avian and Pandemic Influenza and on the standards and guidelines of the World Organization for Animal Health, WHO, the World Trade Organization, and the North American Free Trade Agreement.
To further enhance regional cooperation for health security, the three countries also have a long-standing informal practice of supporting cross-border "collaboration and assistance" (IHR, article 44) by sharing notifications to WHO of potential Public Health Emergencies of International Concern (IHR, article 6) and routine technical reports for the purposes of public health follow-up.
P1.2 The State can demonstrate that it has adjusted and aligned its domestic legislation, policies and administrative arrangements to enable compliance with IHR
Evidence that IHR implementation has been effective in Canada
An internal capacity assessment in 2010-validated by legal counsel in 2015-found that Canadian legislation and regulations were sufficient for IHR compliance and therefore no changes were recommended for IHR implementation. However, Canada understands that the public health landscape is constantly evolving. Through routine performance evaluation processes, and occasional independent investigations, governments in Canada identify and address gaps and weaknesses in existing government instruments.
The Quarantine Act, updated in 2005, serves as a good example of this. Since its implementation, several public health events have underscored challenges regarding the act and increased expectations for federal preparedness and response. Through the Border and Travel Health Modernization Initiative - an opportunity to refresh Canada's approach, strengthen collaboration, and better support compliance with the IHR-Canada is reviewing its legislation and policy, as well as other instruments, and adapting its approach to reflect a changing reality.
Certain policy and administrative arrangements have also been made to improve compliance with the IHR. Following a large Salmonella outbreak in 2014, the Public Health Agency of Canada, the Canadian Food Inspection Agency and Health Canada developed a joint protocol for IHR communications related to food safety issues. The protocol describes how Health Portfolio partners share information with the International Food Safety Authorities Network and WHO during an event with international implications. The protocol also outlines roles and responsibilities and describes reporting mechanisms and requirements to address duplication of effort and to better align messages.
Similarly, protocols have been put in place with the IHR National Focal Point to align reporting requirements in the event that Health Canada's Radiation Protection Bureau reports a real or potential nuclear emergency to the International Atomic Energy Agency under the Convention on Early Notification of a Nuclear Accident.
Best practices, challenges, gaps and recommendations
The Public Health Agency of Canada was created in 2004-with input from provinces, territories, stakeholders, and Canadians-in response to growing concerns about the capacity of Canada's public health system to anticipate and respond to public health threats. The Agency and the Chief Public Health Officer of Canada provide a focal point for federal leadership in managing public health emergencies.
Several provinces (including British Colombia, Ontario, and Quebec) have created their own leadership mechanisms for public health events and emergencies. These centres of expertise for managing public health risks and threats have improved Canada's capacity and leadership in both domestic and international health security. This multi-level arrangement can be viewed as a best practice in a federated state, such as Canada, that relies on strong multi-sectoral engagement and collaboration.
The Agency uses a results-based management approach to support its public health interventions.
As part of this approach, the Agency incorporates specific indicators from the Joint External Evaluation tool and the IHR annual monitoring tool into its regular planning and reporting process. This approach promotes accountability and comparability for delivering essential public health functions. Further, it supports priority-setting and resource allocation, and reinforces the concept that IHR implementation is fully integrated into public health planning and reporting in Canada. The Agency and all other federal departments must report annually to the Canadian public on plans and priorities, expected results, expenditure plans, and performance measurement.
In Canada, implementation of the IHR is enabled through broad health and emergency management legislation. This is supplemented by policy and administrative instruments and accompanied by public health or health-related provincial and territorial legislation. No major legislative gaps have been identified that might prevent the full implementation of the IHR. Canada nonetheless recognizes that policy and administrative instruments may need to be developed or revised to:
- Enable better coordination between legal frameworks
- Strengthen or clarify roles and responsibilities
- Improve performance of activities supporting IHR implementation
- Help address action items resulting from this Joint External Evaluation
P2: International Health Regulations coordination, communication and advocacy
Joint external evaluation target: The effective implementation of the International Health Regulations 2005 (IHR) requires multi-sectoral/ multidisciplinary approaches through national partnerships for effective alert and response systems. Coordination of nationwide resources, including the sustainable functioning of a National IHR Focal Point, which is a national center for IHR communications, is a key requisite for IHR implementation. The National Focal Point should be accessible at all times to communicate with the World Health Organization (WHO) IHR Regional Contact Points and with all relevant sectors and other stakeholders in the country. States Parties should provide WHO with contact details of National Focal Points, continuously update and annually confirm them.
Level of capability in Canada
In July 2005, the Public Health Agency of Canada was designated as the IHR National Focal Point for Canada. It is comprised of an IHR Program team and an IHR Operations function and is supported by the Health Portfolio Operations Centre Watch Office (Watch Office). The Agency performs the four mandatory functions of the National Focal Point as required under the IHR and several additional activities outlined in the WHO National Focal Point Guide.
Canada regularly validates the capacity of its National Focal Point. This is done through informal internal monitoring and assessment, twice yearly communication tests conducted by the WHO Regional Contact Point, and the WHO annual self-assessment.
The success of IHR implementation in Canada relies on ongoing collaboration among relevant sectors on issues of shared responsibility. Several collaborative groups meet regularly to ensure Canada fulfills its IHR responsibilities. One example is the IHR Implementation Working Group, made up of technical and policy experts from the Health Portfolio and other relevant federal departments.
There is also a network of IHR Champions in relevant federal, provincial and territorial government departments. These ensure that the IHR are reflected in regular operational activities and policy development processes. When required, existing federal/provincial/territorial governance mechanisms, task groups and emergency response plans are leveraged to support IHR implementation.
Indicators
P.2.1 A functional mechanism is established for the coordination and integration of relevant sectors in the implementation of IHR
Structure of Canada's IHR National Focal Point
Canada's IHR National Focal Point coordinates the implementation of the IHR on behalf of the Government of Canada. IHR implementation is shared by federal, provincial and territorial governments. The IHR National Focal Point is an implementation hub, providing advice, policy recommendations, advocacy and training, and stakeholder outreach. It also coordinates IHR monitoring and evaluation activities, including annual reporting to the World Health Assembly on behalf of the Government of Canada.
The Watch Office assumes the operational function of the IHR National Focal Point through around-the-clock service as a communications hub. Specifically, the Watch Office coordinates urgent communications concerning the implementation of IHR articles 6 to 12. The team provides critical situational awareness by gathering, organizing and redistributing stakeholder information on public health threats and risks. They also maintain internal tools for IHR communication, such as protocols, distribution lists, and templates.
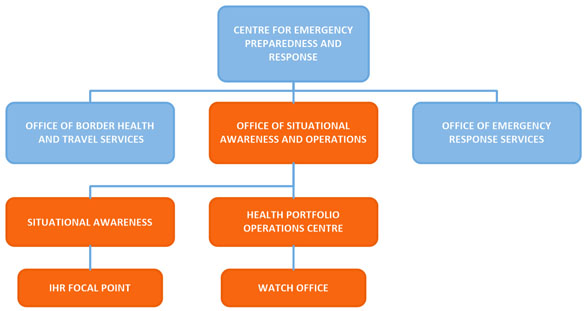
Figure 1: IHR National Focal Point organizational structure - Text description
Figure 1 figure depicts the organizational structure of Canada's IHR National Focal Point. At the top of the organizational chart is the Centre for Emergency Preparedness and Response. The Centre is comprised of three offices: the Office of Border Health and Travel Services, the Office of Emergency Response Services, and lastly the Office of Situational Awareness and Operations. Within the Office of Situational Awareness and Operations there are two key sections to note: Situational Awareness and the Health Portfolio Operations Centre. The IHR National Focal Point falls under the Situational Awareness section and The Watch Office falls under the Health Portfolio Operations Centre. The Office of Situational Awareness and Operations and the areas that stem from it are all coloured in orange to indicate that these areas work together to deliver IHR NFP functions and services. All other teams are displayed in the colour blue.
The Watch Office program achieves 24-hour coverage using two teams: day-time watch officers and after-hours duty officers. During an event or emergency, a dedicated event watch officer is assigned to monitor and triage all event-related operational communications. An IHR technical advisor, generally a public health or health professional, is also available to assist the National Focal Point with assessing and reporting events (using the Annex 2 decision instrument), and with other activities. Advisors are accessible both during and outside regular business hours. The NFP also regularly consult program staff with specific disease expertise. The Vice President of the Health Security Infrastructure Branch (Public Health Agency of Canada) provides oversight on IHR implementation as the IHR Responsible Person in Canada.
IHR National Focal Point staff work closely with stakeholders to align program, policy, operational, legal, and privacy considerations to ensure that Canada continues to meet its assessment and reporting obligations under the IHR.
IHR Champions are designated points of contact in relevant federal, provincial and territorial government departments. They are familiar with Canada's obligations under the IHR and promote and support IHR implementation within their jurisdictions. IHR Champions act as a conduit and contact point for regular information exchange between the IHR National Focal Point and other government stakeholders in Canada.
Collaboration among multiple sectors is facilitated by a working group composed of technical and policy experts from the Health Portfolio and other relevant federal departments. The group meets regularly to support initiatives related to the implementation of the IHR in Canada. It has contributed to a variety of implementation activities, including Canada's Joint External Evaluation, annual self-assessment reports to the World Health Assembly, and other international capacity building activities. The working group does not facilitate collaboration during health emergencies.
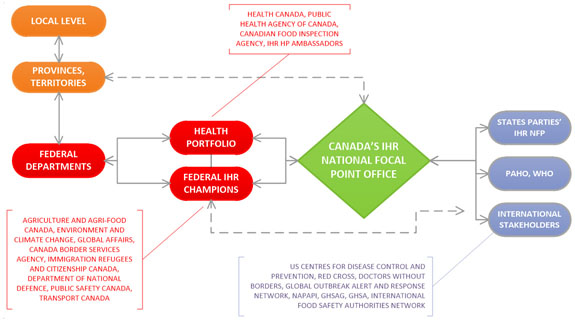
Figure 2: Coordination and information flow between Canada's National Focal Points and other sectors - Text description
Figure 2 diagram illustrates the flow of information and the coordination between Canada's National Focal Point and other sectors. In the top left corner in orange are two ovals representing jurisdictions across Canada. The first oval is the Local level and there is a two-headed arrow connecting it to the oval for Provinces/Territories below it, indicating a 2 way flow of information. The Provinces/Territories oval has a 2-headed dotted arrow connecting it to Canada's IHR National Focal Point Office in the centre of the diagram in a green diamond, and another 2-headed arrow connecting it to Federal Departments below it in a red oval, emphasizing the multi jurisdictional and multi-sectoral coordination that occurs.
To the right of the Federal Departments oval are two additional red ovals. The top one represents the Health Portfolio and the bottom one is Federal IHR Champions. The Health Portfolio is comprised of Health Canada, Public Health Agency of Canada, Canadian Food Inspection Agency and Health Portfolio IHR Ambassadors. Federal IHR Champions include representatives from: Agriculture and Agri-food Canada; Environment and Climate Change Canada; Global Affairs Canada; Canada Border Services Agency; Immigration, Refugees and Citizenship Canada; Department of National Defence; Public Safety Canada; and Transport Canada. There are 2-way arrows connecting the 3 Federal ovals, as well as an arrow leading from these federal entities to the IHR National Focal Point Office.
On the right side of the diagram are 3 blue ovals representing States Parties IHR NFP; PAHO / WHO; and International Stakeholders. International Stakeholders include US Centres for Disease Control and Prevention, Red Cross, Doctors Without Borders, Global Outbreak Alert and Response Network, NAPAPI, GHSAG, GHSA, International Food Safety Authorities Network. There are double sided arrows depicting information flow to/from these international bodies to Canada's IHR National Focal Point Office. There is also a double-sided dotted arrow leading back from these international groups to the Federal IHR Champions.
The Public Health Agency of Canada uses existing governance mechanisms to update partners in other relevant sectors on Canada's IHR-related activities. These mechanisms include the Pan-Canadian Public Health Network, the Council of Chief Medical Officers, the Public Health Infrastructure Steering Committee and the Communicable and Infectious Disease Steering Committee.
In addition to these day-to-day coordination mechanisms, Canada maintains several national emergency response plans (see section R1: Preparedness) and mutual aid agreements to improve response capacity (see sections R2: Emergency response operations and R4: Medical Countermeasures and personnel deployment).
Standard operating procedures, guidance documents and tools
As a best practice, Canada's IHR National Focal Point has developed a comprehensive standard operating procedure with two stand-alone components. One is a high-level strategic overview of Canada's IHR National Focal Point. It outlines its mandate and functions, stakeholder roles and responsibilities, and communication and coordination processes.
The other component is a detailed technical Guidebook for IHR Assessment and Reporting at the Federal (national) Level. This Guidebook supports the Health Portfolio program areas and technical experts in fulfilling their IHR duties to identify, assess, and notify WHO of certain public health events and other reporting requirements. It also includes useful information for all domestic stakeholders with IHR assessment and reporting responsibilities.
The National Focal Point also develops protocols, procedures, process flow maps, and templates to facilitate IHR communications and coordination. This includes the development of a guideline for sharing International Health Regulations notifications of events in Canada with the Council of Chief Medical Officers of Health and IHR Champions.
Individual Health Portfolio programs and other government departments are responsible for developing their own internal processes and procedures for detection, assessment, and reporting public health events, and for communicating relevant events to the IHR National Focal Point.
The IHR National Focal Point has developed additional communication tools to build an IHR community of practice in Canada. These include:
- Online educational resources for basic IHR understanding
- Training courses that provide in-depth operational knowledge and practice for IHR experts within the Health Portfolio
- Presentations to increase awareness of the IHR requirements and strengthen collaboration between sectors
Evaluation and testing of National Focal Point functions
Canada has confirmed its capacity to deliver the four mandatory core functions of a National Focal Point outlined in the IHR and WHO's guidance for National Focal Points in annual self-assessment reports to the World Health Assembly. In the 29th Pan American Sanitary Conference information document on IHR implementation, Canada is recognized as one of 12 States Parties in the Americas that have consistently submitted a State Party Annual Report since the requirement was instituted in 2011.
Canada's IHR National Focal Point performance is regularly monitored and assessed on an informal basis. For example, employees continually monitor the quality and timeliness of IHR communications. The team meets regularly to discuss operational issues, to review and revise internal IHR-specific processes, and to discuss ways to improve services.
Protocols are updated annually and as required. In addition, the National Focal Point staff host an on-going cycle of training and refresher sessions to raise awareness of IHR obligations and to ensure program standards are met. It also conducts regular outreach and provides training to other stakeholder groups (see D3: Reporting).
IHR coordination and communications are also reviewed following a response to an event or emergency. The results of these reviews inform the adjustment of internal protocols, procedures and practices to ensure they are suited to operational realities and emerging challenges.
To ensure the effectiveness of IHR communication functions, the Pan American Health Organization (PAHO), as the WHO IHR Regional Contact Point, conducts twice yearly tests with the designated IHR National Focal Point of States Parties in the Region. Canada's IHR National Focal Point has scored highly on all recent tests. No gaps or incidents of miscommunication have been identified. Nonetheless, these communication tests provide a valuable opportunity for the National Focal Point to review its internal processes and procedures, and to address any issues or deficiencies.
The Public Health Agency of Canada evaluated its emergency preparedness and response program to assess the program's core activities, including relevant IHR National Focal Point activities.
Best practices, challenges, gaps and recommendations
Over the past few years, Canada's IHR National Focal Point has focused efforts on building a foundation for its structure and processes within the Health Portfolio. As a result, Canada has a well-established and fully functional National Focal Point with the capacity to connect with WHO and partners for urgent IHR-related communications.
As a best practice, dedicated staff have been trained and made available to deliver on the National Focal Point's mandatory functions. Guidance documents and tools have also been developed to support these functions. One of the National Focal Point's key strengths is its streamlined approach to coordinating IHR-related communications and information flows.
IHR implementation is a shared responsibility in Canada. Consequently, the National Focal Point plans to strengthen partnerships between sectors by formalizing and standardizing collaboration and communication with federal, provincial and territorial stakeholders, including IHR Champions.
To do this, the National Focal Point will draw on the accumulated experience of the many sectors involved to improve IHR monitoring and evaluation activities, which include the annual reporting process. It will also keep stakeholders updated on implementation progress and establish clear links between health emergency management functions and IHR Champions in order to leverage parallel knowledge and expertise.
Ongoing outreach and training across government on IHR obligations and processes will therefore be key to ensuring that Canada continues to meet its requirements for IHR coordination, communications and advocacy. By building a strong domestic network or community of practice, the National Focal Point is laying the groundwork for open dialogue and information exchange among federal, provincial and territorial partners.
Canada's National Focal Point will also continue to work with PAHO/WHO, the United States, Mexico, and other partners on joint National Focal Point strengthening initiatives. Examples include peer-to-peer exchanges, training opportunities, and the development of valuable resources to help strengthen core capacities related to National Focal Point functions.
These opportunities foster a global community of practice and encourage open communications among National Focal Points. The result is more efficient and effective IHR-related communications within the Americas and beyond. This collaboration helps bolster overall health security and allows Canada to apply lessons learned from other countries to strengthening its National Focal Point capacities.
P3: Antimicrobial resistance
Joint external evaluation target: Support work being coordinated by the World Health Organization, the Food and Agriculture Organization of the United Nations, and the World Organization for Animal Health to develop an integrated global package of activities to combat antimicrobial resistance. The package spans human, animal, agricultural, food and environmental aspects (i.e. a One Health approach). These activities include:
- Each country having its own national comprehensive plan to combat antimicrobial resistance;
- Strengthening surveillance and laboratory capacity at the national and international level following agreed international standards developed in the framework of the Global Action plan while considering existing standards;
- Improving conservation of existing treatments and collaboration to support the sustainable development of new antibiotics, alternative treatments, preventive measures and rapid, point-of-care diagnostics, including systems to preserve new antibiotics.
Level of capability in Canada
In September 2017, the Government of Canada released a new framework, Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action. The Framework strengthens Canada's ability to combat the risks of antimicrobial resistance (AMR) in a coordinated and effective way
to minimize the impact of AMR, and to ensure that antimicrobials will continue to be an effective tool in protecting the health of Canadians. The Framework is grounded in a One Health approach, and developed with experts from the health, public health, veterinary and agriculture and agri-food sectors. Its four components are surveillance; infection prevention and control; stewardship; and research and innovation.
Addressing the threat of AMR in Canada requires the involvement of federal, provincial and territorial governments, health professionals, academia, industry, professional organizations (human and animal stakeholders) and the public. These groups must collaborate, coordinate and leverage activities across sectors to minimize duplication and to create effective, sustainable solutions.
AMR governance to guide the development of the Pan-Canadian Framework and Action Plan has three tiers: a Deputy Minister Champion Committee, an AMR Steering Committee, and four task groups (one for each of the Framework components). This structure has links to national health sector decision-making groups, such as the Public Health Network Council, the Council of Chief Medical Officers of Health, and the Conference of Deputy Ministers of Health. It also links to national agriculture sector committees through the Council of Chief Veterinary Officers, assistant deputy minister level regulatory and policy committees, and the federal, provincial and territorial ministers of agriculture. A federal interdepartmental committee (with representation from 11 departments and agencies) provides overall strategic direction and leadership for the Canadian response to AMR and for Canada's contribution to the international AMR agenda.
Canada has many surveillance systems in place to detect many of the AMR pathogens prioritized by the WHO. These systems include the Public Health Agency of Canada's nine national surveillance programs that track antimicrobial use (AMU) and AMR in both humans and animals. The data collected from these programs informs research and policy.
The Canadian Antimicrobial Resistance Surveillance System produces an annual report using information from national surveillance programs. Canada also has designated laboratories that can detect and report on AMR. One of these is the National Microbiology Laboratory, which is a Level 4 reference laboratory.
Each province and territory has accredited public health laboratories that can conduct AMR detection testing and submit isolates to the National Microbiology Laboratory for serotyping or susceptibility testing, and whole genome sequencing.
The Public Health Agency of Canada (PHAC) is the national coordinator for monitoring the incidence of AMR in bacterial isolates from human infections and in enteric bacteria along the food chain. The Canadian Nosocomial Infection Surveillance Program is responsible for clinical surveillance. The Program encompasses 65 acute-care hospitals which serve as sentinel surveillance sites for many healthcare associated infections, including infections due to Clostridium difficile and antibiotic-resistant organisms such as methicillin-resistant Staphylococcus aureus. Canada also collects some data on antibiotic-resistant organisms in community settings, including long-term care facilities.
For surveillance along the food chain, the Canadian Integrated Program for Antimicrobial Resistance Surveillance collects AMU data (national sales data, and farm-level data), and gathers cecal and other samples for AMR testing from farms, abattoirs, and retail food isolates. It also does AMR testing on clinical Salmonella isolates from animals and humans. Surveillance data from these systems is aggregated and publicly reported.
The delivery of health care in Canada is largely the responsibility of the provinces and territories. The federal government publishes evidence-based national guidance on infection prevention and control to inform and complement provincial and territorial guidelines, standards and protocols. This guidance is also used by different jurisdictions and facilities in the development of their policies and protocols.
In 2016 PHAC published Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Healthcare Settings. PHAC is currently updating the 2002 version of the national guidelines on the prevention and control of occupational infections in health care. In addition to infection prevention and control policies, standards and plans for their health care facilities, most provinces and territories have access to designated infection control professionals on-site or through teaching hospitals.
The availability of isolation units in Canadian hospitals is high, although their capacity varies. Canada also has ad hoc measures in place to assess the effectiveness of infection prevention and control measures and share results.
As with health care, animal health care is largely the responsibility of the provinces and territories. Health Canada approves antimicrobials for sale for use in animals, whereas the provinces and territories control the distribution of antimicrobials and regulate the practice of veterinary medicine, At the farm level, National Biosecurity Standards and Biosecurity Principles, developed by the Canadian Food Inspection Agency (CFIA), in collaboration with producer organizations, provincial and territorial governments and academia, complement voluntary on-farm food safety programs.
The 2015 Federal Action Plan on Antimicrobial Resistance and Use in Canada: Building on the Federal Framework for Action includes commitments to strengthen the promotion of the appropriate
use of antimicrobials in human and veterinary medicine, and to continue to strengthen the regulatory framework for veterinary medicines and medicated feeds. Health care facilities across Canada have implemented antimicrobial stewardship programs and identified best practices. Veterinary Oversight of Antimicrobial Use – A Pan-Canadian Framework for Professional Standards for Veterinarians, guidelines and standards are in place to support the prudent use of antimicrobials in animals.
Health Canada monitors antibiotic sales and the number of prescriptions for human drugs using data collected from community pharmacies and hospitals, which it purchases from IMS Health Canada Inc. PHAC analyzes this antibiotic use data. Pilot studies are underway to improve our understanding of prescription practices for certain classes of antibiotics and select indications.
Health Canada regulates and approves veterinary drugs. Policies and regulations outlined by Health Canada related to feed are implemented and regulated by CFIA. PHAC analyses data provided by the Canadian Animal Health Institute on the annual volume of veterinary antibiotics distributed for sale in Canada. Additionally, Canada collects data on antimicrobial use at the farm level through the Canadian Integrated Program for Antimicrobial Resistance Surveillance sentinel farm program.
Health Canada maintains a Prescription Drug List, which is a list of medicinal ingredients that, when found in a drug, require a prescription for use in humans and animals. All systemic antibiotics in humans require a prescription in Canada, and all medically-important antibiotics for use in animals will require a prescription as of December 1, 2018.
Indicators
P.3.1 Antimicrobial resistance detection
National AMR plans
In September 2017, the Government of Canada released Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action. The Pan-Canadian Framework provides an overarching policy frame that lays out strategic goals and guiding principles to address antimicrobial resistance (AMR) in Canada. Specifically, it outlines the need for action across all jurisdictions and implicated sectors in the areas of: surveillance; stewardship; research and innovation; and infection prevention and control.
The Pan-Canadian Framework builds on previous plans that were developed to address AMR. In October 2014, the Government of Canada released Federal Action Plan on Antimicrobial Resistance and Use in Canada: Building on the Federal Framework for Action, which maps out a coordinated, collaborative federal approach to responding to the threat of AMR. In March 2015, the Federal Action Plan on Antimicrobial Resistance and Use in Canada: Building on the Federal Framework for Action was published. It identifies concrete steps to be taken by key federal departments and agencies to achieve the goals of the federal framework, including efforts to establish and strengthen surveillance systems.
Surveillance programs for AMR bacteria in Canada
Surveillance is a shared jurisdictional responsibility in Canada. There are data sharing agreements in place between federal, provincial and territorial partners. Surveillance is also one of the four pillars of the Pan-Canadian Framework. There are multiple surveillance systems in place at different levels of government in Canada that collect data on anti-microbial resistance and anti-microbial use (AMU) in human and animal settings, such as hospitals, community settings, agricultural settings and farms. The data from these systems is used to inform and update AMU and infection control policies.
- The Canadian Antimicrobial Resistance Surveillance System provides an integrated picture of AMR and AMU in Canada based on surveillance data from laboratory reference services and PHAC's surveillance systems that track identified priority organisms and AMU in humans and animals.
- The Canadian Integrated Program for Antimicrobial Resistance Surveillance monitors trends in AMU and AMR in selected bacterial organisms from human, animal and food sources across Canada. The program is based on several representative and methodologically unified surveillance components. These are linked to examine the relationship between antimicrobials used in food-animals and humans and the associated human health impacts. This information supports the creation of policies to control AMU in hospital, community and agricultural settings and the identification of appropriate measures to contain the emergence and spread of resistant bacteria between animals, food and people in Canada.
- In Canada, the list of nationally notifiable diseases for humans covers infectious diseases that have been identified by the federal government and all provinces and territories as priorities for monitoring and control efforts-this includes resistant microbes. Through the Canadian Notifiable Disease Surveillance System, provinces and territories voluntarily submit annual notifiable disease data which are used to produce national disease counts and rates.
The list of nationally notifiable diseases is revised periodically. Since 1987, all decisions about what diseases to include in the list are based on federal, provincial and territorial consensus, using set criteriaFootnote 1. Data from the Canadian Notifiable Disease Surveillance System is available to the public through Notifiable Diseases Online.
Laboratory capacity for AMR detection
The National Microbiology Laboratory is Canada's National Reference Laboratory and the designated laboratory for many of the Public Health Agency of Canada's AMR surveillance programs. The Laboratory is Canada's only Level 4 reference laboratory and is accredited by the World Organisation for Animal Health for Salmonella. The Laboratory validates its data using internal quality controls-control strains with a known minimal inhibitory concentration range and negative control. The Laboratory holds accreditation to ISO 15189 and ISO 17025 for some, but not all, tests. Many tests performed at the Laboratory are verified annually through participation in external proficiency tests or inter-laboratory comparisons.
Provinces and territories have public health laboratories, many of which can conduct AMR detection testing, or alternatively, submit isolates to the National Microbiology Laboratory for serotyping or susceptibility testing. The AMR pathogens for which laboratories can test vary from facility to facility due to surveillance priorities and testing methods within each province and territory.
The CFIA operates laboratories for pathogen testing in food, animals, and animal feed. It types every pathogenic isolate for the presence of AMR markers using whole genome sequencing techniques. All CFIA laboratories are ISO 17025 accredited and routinely participate in external proficiency tests on inter-laboratory verification.
Clinical laboratories in Canada are certified through a variety of accreditation programs and there are several external quality assurance bodies in Canada and in North America to ensure quality testing and reporting of data. Routine antimicrobial susceptibility testing methods are aligned with the Clinical and Laboratory Standards Institute and/or the European Committee on Antimicrobial Susceptibility Testing Guidelines. Automated testing methods or testing on new antimicrobials follow the U.S. Food and Drug Administration guidelines, if available. All hospital and provincial clinical laboratories are certified through the College of American Pathologists accreditation program.
AMR reporting activities
The annual Canadian Antimicrobial Resistance Surveillance System report integrates AMR and AMU data from human and food chain surveillance systems. This includes information on surveillance data, initiatives and gaps, and the list of priority resistant pathogens to monitor on a national level in humans and animals. Data obtained from the Canadian Antimicrobial Resistance Surveillance System, the Canadian Integrated Program for Antimicrobial Resistance Surveillance, and the Canadian Nosocomial Infection Surveillance Program are communicated to stakeholders on request, and also made available to the public online.
All certified clinical laboratories in Canada report data on individual patients to physicians for patient care, while some provincial and private animal health laboratories submit clinical and non-clinical Salmonella isolates to the National Microbiology Laboratory for serotyping. Some provinces collect specific AMR pathogen information from hospital laboratories and animal health laboratories. However, this varies from province to province.
P.3.2 Surveillance of infections caused by antimicrobial-resistant pathogens
Clinical surveillance programs
- The Canadian Antimicrobial Resistance Surveillance System collects and reports information on human and animal AMU from PHAC's surveillance systems, and from laboratory reference services. The System also collects information on antimicrobial resistant organisms in community settings, including long-term care facilities.
- The Canadian Nosocomial Infection Surveillance Program collects national data on various healthcare associated infections, including infections from antimicrobial resistant organisms. The Program is the only national surveillance system for identifying and monitoring rates and trends in national and regional hospital-associated infections. These include antimicrobial resistant organisms and their strain types and resistance patterns, some of which have been identified as priorities for AMR surveillance by the Pan-Canadian Public Health Network and Communicable and Infectious Disease Steering Committee's Antimicrobial Resistance Surveillance Task Group.
As of 2017, the Canadian Nosocomial Infection Surveillance Program conducts surveillance on:
- Hospital-associated Clostridium difficile infections
- Methicillin-resistant Staphylococcus aureus infections, including healthcare- and community-associated methicillin-resistant Staphylococcus aureus and methicillin-resistant Staphylococcus aureus bacteremia
- Vancomycin-resistant enterococci infections
- Carbapenemase-producing organisms, including carbapenemase-producing Enterobacteriaceae and carbapenemase-producing Acinetobacter
- Antimicrobial resistant organisms related to central line-associated bloodstream infections in intensive care units and hip and knee surgical site infections
- There are 551 acute-care hospitals in Canada, according to information from the Canadian Institute for Health Information. As of December 2016, the Canadian Nosocomial Infection Surveillance Program was conducting surveillance in 65 acute-care hospitals in 10 provinces in Canada. Of these, 13 are large, acute, tertiary care hospitals with more than 500 beds; 32 of the acute-care hospitals are intermediate size (201 to 500 beds) while the remaining 20 are smaller acute-care facilities with less than 200 beds.
- The only animal clinical pathogen that the Canadian Integrated Program for Antimicrobial Resistance Surveillance monitors is Salmonella, which is received from passive surveillance of veterinary diagnostic laboratories across Canada. National representation of human clinical Salmonella is obtained from provincial and territorial public health laboratories. Human clinical specimens of Campylobacter are collected for AMR testing through a partnership with a sentinel surveillance program called FoodNet Canada.
In addition to participating in the Canadian Nosocomial Infection Surveillance Program, many health care facilities have their own surveillance and monitoring programs in place. For example, Manitoba reports that all of the five regional health authorities and the mental health centre conduct surveillance for AMR organisms. Alberta, British Columbia, and Ontario report that all of their acute-care hospitals conduct surveillance for AMR pathogens in humans.
Quebec has a provincial surveillance program (Available in French only) for nosocomial infections, including several AMR pathogens. The participation of short-term hospitals in these monitoring programs is mandatory. The Yukon Hospital Corporation tracks rates of methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci and Clostridium difficile across the territory's three hospitals. The Stanton Territorial Hospital serves as the sentinel site for the Northwest Territories.
Animal and food chain surveillance programs
- According to the Archived: 2016 Census of Agriculture, there are 77,594 livestock and poultry farms in Canada, as classified by the North American Industry Classification System. Of these, 57,802 are cattle, sheep, goat, pig, or poultry (chicken and turkey) farms. From this total, 309 are selected to represent their respective commodities, acting as Canadian Integrated Program for Antimicrobial Resistance Surveillance sentinel sites for the surveillance of AMR enteric bacteria in livestock on farms. As of May 2017, there were 90 sites for pigs, 129 for broiler chickens, 78 for turkeys, and 12 for beef cattle (large feedlots). Samples at these sites are collected to isolate foodborne enteric bacteria (E. coli, Salmonella, and Campylobacter).
- The Canadian Integrated Program for Antimicrobial Resistance Surveillance also includes national data from cecal samples obtained from beef, swine and chickens at federally registered slaughter facilities across Canada.
- The Canadian Animal Health Surveillance Network is a collaborative federal-provincial-academic network that links veterinary laboratories across the country to collect animal surveillance data. The network also serves as a surveillance system for animal disease threats to animal and human health and to the security of the food supply.
P.3.3 Healthcare associated infection prevention and control programs
Infection prevention and control in the context of AMR focuses on non-antimicrobial strategies that prevent infections in human healthcare, community, veterinary and agriculture settings. In settings where healthcare is delivered, infection prevention and control programming is essential. This applies particularly to hospitals due to the risk of acquiring hospital-associated infections such as:
- Methicillin-resistant Staphylococcus aureus
- Carbapenem-resistant Enterobacteriaceae
- Multidrug-resistant Enterobacteriaceae
- Multidrug-resistant Candida auris
- Vancomycin-resistant enterococci
Infection prevention and control is another pillar of the Pan-Canadian Framework.
Healthcare associated infections surveillance and monitoring
The federal government conducts healthcare associated infections surveillance through the Canadian Nosocomial Infection Surveillance Program, which had 65 active sites as of December 2016. The Program sites share surveillance information (reports, data) through the Program network and with provincial and territorial partners (Ministers of Health) and the federal government to identify emerging trends and outbreaks. The federal government publishes Program reports on its website. Researchers present Program data at conferences, such as the annual Association of Medical Microbiology and Infectious Disease [Canada] meeting, and they publish papers on the data in peer-reviewed journals.
Provinces and territories also monitor high-risk groups for healthcare associated infections. For example, New Brunswick reports that one regional health authority conducts screening for methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci in dialysis and extended care units every six months, which will soon be accelerated to every three months. One facility conducts extended-spectrum beta-lactamase screening in dialysis units every three months. The other regional health authority screens high-risk admissions for targeted antimicrobial resistant organisms, while in both health authorities, neonatal units participate in the Canadian Neonatal Network, reporting positive blood and cerebrospinal fluid cultures.
Healthcare-associated infection plans and programs
The federal government develops national evidence-based guidelines and makes them publicly available to healthcare settings. The guidelines inform and complement guidance, standards and protocols developed by provinces and territories, regional health authorities and healthcare facilities, and guidance documents developed by other organizations.
The federal government has published a series of Infection Control Guidelines to help healthcare organizations, infection control professionals and other healthcare providers develop policies and procedures related to infection prevention and control practices in healthcare settings. Within this series are guidelines on Nosocomial and Occupational Infections that address specific diseases, including Clostridium difficile in both acute-care and long-term care facilities, and Carbapenem-resistant Gram-negative bacilli. There is also guidance on Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Healthcare Settings.
The federal government manages an expert advisory body for infection prevention and control, and engages with provinces, territories and stakeholders. Policy activities take place at the AMR and healthcare associated infections interface. For example, the Canadian Nosocomial Infection Surveillance Program works with the Canadian Hospital Epidemiology Committee of the Association of Medical Microbiology and Infectious Disease Canada on AMR pathogens that should have data collected at the hospital level.
Other groups develop standards informed by federal guidance:
- Infection Prevention and Control Canada and the Association of Medical Microbiology and Infectious Disease Canada are two groups that are formally recognized as "liaison member organizations" of PHAC's Infectious Diseases Prevention and Control external advisory body.
- Canadian Standards Association develops and maintains standards for infection prevention and control, sterilization, medical devices, and health care facility infrastructure.
- Accreditation Canada develops required operational practices for infection prevention and control.
- Canadian Patient Safety Institute has developed a number of tools for infection prevention and control, including a "Getting Started Kit", videos, and hand hygiene programming. The Institute collaborates with PHAC and other partners on the annual STOP! Clean Your Hands Day campaign.
Provinces and territories design and implement their own infection prevention and control strategies, which vary in scope and coverage. For example, in 2006, the Quebec ministry of health and social services (MSSS) established a reference framework for healthcare establishments for the prevention and control of nosocomial infections that defines procedures for combating nosocomial infections. The framework was updated in 2017.
In addition, every health care facility in Quebec must develop a nosocomial infection prevention program covering six components:
- Monitoring of nosocomial infections
- Policies, procedures and support measures
- Education and training
- Evaluation
- Communication and information
- Management of outbreaks
The content of the program must be adapted by each institution according to its size, mission, activities, and the priorities arising from local/regional epidemiology.
The federal government, provinces and territories have guidelines for protecting health care workers from occupational infections, including:
- Canada's national guideline on the Prevention and Control of Occupational Infections in Health Care. The guideline, which is being updated, supports collaboration between occupational health and infection control programs. It focuses on infectious diseases that are known to have been transmitted from person-to-person through occupational exposure, and on infections that may occur during the delivery of health care.
- Two Ontario guidance documents on the protection of health care workers from healthcare associated infections, which elaborate on the Ontario Occupational Health and Safety Act and regulations. Best Practices for Infection Prevention and Control Programs in Ontario explains the importance of adhering to the act and regulations and covers the use of personal protective equipment. Regulations pertaining to the proximity of food and drink to infectious materials; needle safety; and ceiling exposure values for biological and chemical agents. Routine Practices and Additional Precautions describes the requirements of the act and regulations and best practices for implementing them.
Trained infection control professionals
Dedicated infection prevention and control resources and programs vary across jurisdictions and centres. Teaching hospitals generally have higher proportions of infection control professionals and infectious disease specialists than community and long-term care settings. Infection prevention and control training is typically facility- or program-based.
British Columbia, Saskatchewan, Nova Scotia, and Newfoundland and Labrador report infection control professionals in all tertiary hospitals. Prince Edward Island has infection control professionals in all health authority hospitals, community hospitals, long-term care facilities, and community programs.
In Quebec, hospitals that provide tertiary care have infectious disease microbiologists assigned to infection prevention and control, some of whom have completed specialized training. Acute-care hospitals in New Brunswick fall within the mandate of the infection prevention and control programs in their regional health authority, including access to infection control professionals. Smaller and medium-sized hospitals, where infection control professionals may not be on site daily, have access to them via telephone or internet.
Hospital isolation units
The Canadian Standards Association Group has standards and codes for the design, construction and renovation of healthcare facilities. The national Infection Control Guideline series and the Routine Practices and Additional Precautions document include general recommendations for isolation units. These recommendations are implemented at the provincial and territorial level.
Most provinces have isolation units or rooms, and several have airborne infection isolation rooms.
For example, in Alberta airborne infection isolation rooms are available and are monitored for function. Contact and droplet protection is provided in isolation rooms (with anterooms), in private patient rooms, and in shared patient rooms using appropriate cohort precautions. Infection control professionals are available to all acute-care hospitals for consultation on management of patients that require isolation. Alberta Health Services has created an isolation reference guide for adults as part of their overall resource manuals for infection prevention and control.
New Brunswick has 111 stand-alone airborne infection isolation rooms located in hospitals of varying sizesFootnote 2. Saskatchewan and Newfoundland and Labrador report that their tertiary hospitals are equipped with isolation rooms.
Assessing the effectiveness of infection prevention and control practices
Many of the infection prevention and control programs at the provincial and territorial level include reporting requirements and/or evaluation components. For example, Quebec, as part of its Ministerial Action Plan 2015-2020 (Available in French only) on the Prevention and Control of Nosocomial Infections, is planning an evaluation of the clinical and economic impact of nosocomial infections and the effects of infection prevention and control measures on these infections in general and specialized care hospitals.
In addition, members of Institut national de santé publique du Québec (Available in French only) [Quebec national public health institute] committees-the Quebec Nosocomial Infections Committee (Available in French only) and the Provincial Committee for Surveillance of Nosocomial Infections (Available in French only) - publish scientific articles on the impact of the guidelines on the rate of nosocomial infections.
Ontario has a Provincial Infectious Diseases Advisory Committee that develops best practices, reports and recommendations on communicable diseases, immunization, infection prevention and control and surveillance. Prince Edward Island and Saskatchewan evaluate their infection prevention and control according to Accreditation Canada requirements.
P.3.4 Antimicrobial stewardship activities
National plans and programs
Antimicrobial stewardship is a focus area of the Pan-Canadian Framework. There are a myriad of other hospital, community and veterinary-based antimicrobial stewardship initiatives in Canada that promote appropriate AMU among those who prescribe and dispense antimicrobials and those who use them. These initiatives include:
- Regulatory frameworks in place to review and approve antimicrobials for human and animal use, most of which have prescription status in Canada. The main regulatory framework (the Food and Drug Regulations, under the authority of the Food and Drugs Act) consists of various regulatory provisions applicable to all drugs sold in Canada, including antibiotics.
- Health Canada monitors the safety of antimicrobials on the market and requests manufacturers to make changes to labelling as needed. The Archived: Protecting Canadians from Unsafe Drugs Act outlines measures to strengthen Health Canada's oversight on the safety of therapeutic products throughout their life cycle; to improve reporting of serious adverse drug reactions and medical device incidents; and to increase regulatory decision-making transparency.
- Many health care facilities nationwide implement antimicrobial stewardship as part of voluntary accreditation standards.
Canadian initiatives that support the prudent use of antimicrobials in humans include:
- A stakeholder-led action plan on antimicrobial stewardship: Putting the Pieces Together: A National Action Plan on Antimicrobial Stewardship.
- Antibiotic resistance information and awareness materials for Canadians and health care professionals.
- Pan-Canadian Public Health Network Communicable and Infectious Disease Steering Committee Task Group on Antimicrobial Use Stewardship.
- Community education program about handwashing and the responsible use of antibiotics, "Do Bugs Need Drugs?"
- Canadian Guidelines on Sexually Transmitted Infections is a resource for primary care and public health professionals for the prevention, diagnosis, treatment and management of sexually transmitted infections.
- Practical tools and information for practitioners, as well as webinars on antimicrobial resistance offered on Antibiotic Awareness Week Canada.
- Choosing Wisely Canada is a campaign to help clinicians and patients engage in conversations about unnecessary tests and treatments, and make smart and effective care choices
Prudent antimicrobial use in animals is supported through a variety of programs, such as:
- A new national framework, Veterinary Oversight of Antimicrobial Use: A Pan-Canadian Framework of Professional Standards for Veterinarians supports provincial and territorial veterinary regulatory bodies in developing regulations, guidelines or bylaws related to veterinarian oversight in the use of antimicrobials. The framework contributes to the development and implementation of uniform regulations across the country.
- The Canadian Veterinary Medical Association's Antimicrobial Prudent Use Guidelines for Beef Cattle, Dairy Cattle, Poultry and Swine. The guidelines are being updated to include small ruminants and companion animals.
- National on-farm biosecurity standards, protocols and strategies developed for all major livestock and poultry groups in collaboration with federal, provincial and territorial governments, producer organizations and academia. These standards are implemented on a voluntary basis by many producer associations and farmers.
- Voluntary on-farm food safety programs designed by federal, provincial and territorial governments, in consultation with food animal producers, are based on recognized standards and best practices and focus on food safety and animal care. These include prudent use of antimicrobials to promote good animal health. Examples include: the Canadian Cattlemen's Association's Verified Beef Production Plus Program; the Canadian Pork Council's Canadian Quality Assurance program; and the Dairy Farmers of Canada's Canadian Quality Milk program and "proAction initiative."
- Chicken Farmers of Canada's Antimicrobial Use Strategy.
- The health sections of the National Farm Animal Care Council Codes of Practice for the care and handling of farm animals are guidelines on appropriate infection prevention and control and treatment of disease.
- Recent changes to the Food and Drug Regulations to increase oversight of antimicrobials available for use in animals. The changes restrict who can import medically-important antimicrobials and prevent the importation of the active pharmaceutical ingredients in medically-important antimicrobials by producers for their direct use in food animals. They also restrict the personal importation of drugs for food-producing animals to those on List B and prohibit medically-important antimicrobial labels from including growth promotion claims.
- Canada has in place legislation and regulations pertaining to import, marketing authorisation, production, distribution, quality control, and prudent use of high-quality veterinary drug products, including antimicrobials. These rules support a system of inspection of the whole drug chain.
During the evaluation of drug submissions for new antimicrobials, there is a requirement for studies or data assessing the risks arising from the potential development and spread of resistance under the proposed conditions of the use of the product.
Antimicrobial drug product inserts and labels give precise information on conditions for use, linked to studies in the drug submission application (compliance with prescribed doses, treatment duration, withdrawal period, and specific warning statements in the event of antimicrobial resistance).
Antimicrobial use monitoring in human medicine
- Canada has initiated several pilot surveys of antimicrobial use, including the Fluoroquinolone Prescribing and Use in Canadian Primary Care Practice survey, which provides a better understanding how the fluoroquinolone class of antibiotics is prescribed in humans. Another initiative is a two-phase study by the Drug Safety and Effectiveness Network to evaluate usage patterns across Canada of fluoroquinolones in select indications where the benefit of using these drugs may be marginal.
- Health Canada monitors antibiotic sales and the number of prescriptions for human drugs, while the Public Health Agency of Canada analyzes antibiotic use data collected from community pharmacies and hospitals and purchased from IMS Health Canada Inc.
- Under the Food and Drugs Act, Health Canada maintains the Prescription Drug List. It is a list of medicinal ingredients that, when found in a drug, require a prescription. A prescription is required for the use of all systemic antibiotics in humans. Some topical antibiotics containing bacitracin, mupirocin, polymyxin B sulfate, or gramicidin do not require a prescription.
Antimicrobial use monitoring in food production, veterinary medicine and the environment
- Since 2006, the Canadian Integrated Program for Antimicrobial Resistance Surveillance has reported data from the Canadian Animal Health Institute on the annual volume of veterinary antimicrobials distributed for sale in Canada. Recent regulatory amendments require manufacturers and importers of veterinary drugs in dosage form that contain an active pharmaceutical ingredient for medically-important antimicrobials to report annually on the total quantity of each drug sold and provide an estimate of the quantity sold for each intended animal species. Individuals who import, compound and sell an active pharmaceutical ingredient for medically-important antimicrobials for veterinary use must also report on an annual basis.
- Most of the newer antimicrobials approved since 2004 for use in animals require a prescription. Canada is working to implement increased oversight by including the remaining medically-important antimicrobials for veterinary use on the Prescription Drug List. As of December 1, 2018, all such drugs for veterinary use will be sold by prescription only.
- The Canadian Integrated Program for Antimicrobial Resistance Surveillance monitors antimicrobial use at the farm level in sentinel sites from swine, broiler chickens, turkey, and is developing plans to monitor in other commodities.
Best practices, challenges, gaps and recommendations
Through the Pan-Canadian Framework for Action on AMR and AMU, Canada has established a foundation to identify steps to address key AMR and AMU challenges. The next phase will focus on creating a Pan-Canadian action plan, which will lay out concrete deliverables, timelines, measurable outcomes, priorities and allow for tracking progress.
The complexity of AMR underscores the need for coordinated action by key actors-governments, private and public partners, and the public-across the human, animal and environmental sectors. Canada has successfully brought together a variety of stakeholders engaged in fragmented activities across multiple sectors to work collaboratively. The goal moving forward is to maintain and enhance these activities.
Data collection from a range of healthcare, community, agriculture and veterinary settings is complex. Canada has developed a strong surveillance infrastructure for AMR. However, surveillance is a shared responsibility and there remain variations in how data are collected, managed and reported by multiple systems at the local, provincial, territorial and federal levels. In addition, resource and infrastructure capacity to collect and analyze data varies. Within this landscape, there is a need to ensure comprehensive data comparison and analysis of AMR and AMU.
Canada has done much to identify gaps related to its surveillance, stewardship, and infection prevention and control activities, as well as in the area of research and innovation (the fourth component of the Pan-Canadian Framework). Canada is committed to enhancing the strong One Health approach it has developed domestically to address AMR, and to leveraging it internationally.
Given the extensive consultations that have recently taken place in Canada during the development of the Pan-Canadian Framework, the recommendations outlined below focus on actions that will strengthen Canada's ability to reduce the risks of AMR in a coordinated and effective manner:
- Strengthen governance structures to generate knowledge and information on AMR and AMU in humans, agriculture and animals through the monitoring, detection and tracking of resistant organisms to develop and monitor interventions.
- Promote, facilitate and measure appropriate AMU in humans and animals to conserve the effectiveness of antimicrobials that are critical to human and animal health, and to limit the development and spread of resistant organisms within and among populations.
- Reduce the need for antimicrobial treatment by promoting infection prevention and control practices to decrease infection rates in healthcare, community and animal settings.
- Support the advancement of research and innovative approaches for the identification, characterization and real-time detection of microorganisms, including resistant bacteria, and the treatment and prevention of infections as well as basic and behavioural research.
P4: Zoonotic disease
Joint external evaluation target: Adopted measured behaviours, policies and/or practices that minimize the transmission of zoonotic diseases from animals into human populations.
Level of capability in Canada
In Canada, provincial and territorial governments and other animal and human health stakeholders routinely work with federal departments to prevent, detect and respond to outbreaks of zoonotic diseases of public health importance. Among these departments are the Public Health Agency of Canada (PHAC), the Canadian Food Inspection Agency (CFIA), Health Canada, and Environment and Climate Change Canada.
In Canada, enteric and non-enteric zoonoses are managed in separate but related streams. (For more information on enteric zoonoses see section P5: Food safety.) Canada is building its animal and human epidemiology, surveillance, response, and laboratory capacity at all levels. The focus is on non-enteric zoonotic diseases in both human and animal populations (including wildlife, pets, livestock and arthropod vectors).
The One Health approach, which is critical to the prevention and control of zoonoses, is generally accepted in Canada. Some national strategies take into consideration the human-animal-environment interface, which drives more and better collaboration across disciplines and sectors.
Domestic animal health is well managed and regulated at the provincial, territorial, and federal levels. Canada's wild animal populations are relatively large and dispersed. Consequently, responsibility for monitoring wildlife is a collaborative effort between several government and non-government organizations. These include provincial and territorial ministries of environment, conservation and natural resources; federal departments (such as Environment and Climate Change Canada, the Department of Fisheries and Oceans, Parks Canada); the Canadian Wildlife Health Cooperative; and Indigenous groups.
Canada has in place a number of well-established non-enteric zoonotic disease surveillance programs for disease occurrence in animal populations, including for West Nile virus, Lyme disease, rabies and animal (primarily avian) influenza. Provincial and territorial governments have primary responsibility to investigate illnesses and outbreaks of zoonotic diseases in humans, with support from relevant federal departments such as PHAC and the CFIA.
Government and non-government partners work together for a coordinated One Health approach. Canada's List of Nationally Notifiable Diseases covers zoonotic diseases in humans, while the CFIA's Federally Reportable, Immediately Notifiable and Annually Notifiable Diseases in Canada covers zoonotic diseases in animal populations.
At all levels of government there are strategies to guide emergency operations centres to coordinate an effective response to public health events, including zoonotic disease outbreaks. (See section R2: Emergency response operations.)
Canada has a sustainable, well-trained veterinary workforce of approximately 13,700 veterinarians. Five accredited colleges of veterinary medicine in Canada offer programs for pre-clinical and clinical study, as well as opportunities for advanced training and post-graduate study in public health and epidemiology. They have active research and teaching programs and operate veterinary diagnostic laboratories. Internship and fellowship programs are available in Canada to graduates of veterinary medicine and courses. These also extend to established professionals, for gaining new skills related to epidemiology, public health, and laboratory diagnosis for zoonoses in animals and people.
Indicators
P.4.1 Surveillance systems in place for priority zoonotic diseases and pathogens
Priorities for national zoonotic disease surveillance and control
The identification and prioritization of zoonotic diseases are brought forward via a number of mechanisms, including public health governance structures, programmatic analysis and decision-making and national technical informal working groups.
The Canadian Notifiable Disease Surveillance System is a federal, provincial and territorial collaboration that enables all levels of public health to share information on nationally notifiable infectious diseases. Provinces and territories use the System to submit annual notifiable disease data, which are used to produce national disease counts and rates. The Notifiable Disease Charts can be used to explore trends since 1924.
The List of Notifiable Diseases provides disease names and years in which they were considered notifiable. Public health departments use the information to monitor, prevent and control the occurrence and spread of all notifiable zoonoses, as well as other diseases and conditions. Non-enteric zoonoses under surveillance in Canada through the Canadian Notifiable Disease Surveillance System include: anthrax, brucellosis, hantavirus pulmonary syndrome, Lyme disease, malaria, plague, rabies, tularemia, viral hemorrhagic fevers, West Nile virus, and yellow fever.
The One Health concept is generally accepted in Canada and the integration of human, animal and environmental factors is regularly considered and applied in policy and program decisions. Pan-Canadian approaches reflected in strategies, such as the National Plant and Animal Health Strategy, the Pan-Canadian Framework on Clean Growth and Climate Change, and the National Wildlife Health Strategy (currently in development), all take into consideration the human-animal-environment interface. Canada also has several governance committees, councils and working groups listed under "Mechanisms in place to identify priorities" below. These bodies review and discuss issues related to One Health (see "Public health governance structures" below).
Several recent events in Canada have provided opportunities to test the skills of both human and animal public health workers in investigating and responding to a non-enteric zoonotic event:
- In September 2016 the CFIA began an investigation, still on-going, into cases of bovine tuberculosis in cattle on a farm in Alberta.
- In July 2016 authorities Archived: detected cases of H5N2 LPAI (low path) on a commercial duck farm in Ontario.
- In April 2015 there was an outbreak of H5N2 HPAI on a commercial poultry farm in Ontario.
- In December 2014 there was an outbreak of H5N2 HPAI on a number of farms in British Columbia.
The most significant lessons learned from these events were:
- Engage relevant organizations quickly.
- Know what information can and cannot be shared.
- Share the right information with the right people.
- Nurture strong, collegial relationships between key players for fast, effective collaboration.
Mechanisms in place to identify priorities
Canada has a number of mechanisms in place to identify priorities for national non-enteric zoonotic disease surveillance and control. These include public health governance structures, programmatic exercises (environmental scans), and informal technical working groups of federal, provincial and territorial experts.
Public health governance structures
Zoonotic diseases can be brought forward for discussion and prioritization (as required), to a number of public health governance structures and informal working groups, including:
- the Council of Chief Medical Officers of Health,
- the Communicable and Infectious Disease Steering Committee,
- the Public Health Network Council,
- the Council of Chief Veterinary Officers,
- the National Farmed Animal Health and Welfare Council,
- PHAC/CFIA Non-Enteric Zoonotic Diseases Steering Committee, and
- Federal Partners in Wildlife Health.
Programmatic exercises
In 2012, PHAC conducted an assessment of non-enteric zoonotic diseases of public health significance with the highest risk of emergence or re-emergence in Canada. Risks were identified according to internationally accepted indices defining disease emergence and other criteria, including:
- Increased incidence in humans
- Increased geographic range
- Status of surveillance systems
- Necessity for inter-sectoral collaboration (between human public health and veterinary public health) in each province and territory
- Necessity for national collaboration in research, surveillance, diagnostic testing or communication and education
This assessment identified four disease priorities:
- Lyme disease and other emerging tick-borne diseases [highest score]
- West Nile virus and other emerging mosquito-borne diseases
- Rabies
- Zoonotic influenza
The findings were finalized and presented to the Communicable and Infectious Diseases Steering Committee of the Public Health Network Council in 2013. They were used to inform areas of future federal, provincial and territorial collaboration on non-enteric zoonoses in Canada.
In addition, the Infectious Diseases Prevention and Control Branch does annual strategic and operational planning exercises to identify emerging risks and develop action plans to address them, such as the Zika virus action.
Technical working groups
Two communities of practice, or informal technical working groups: the West Nile Virus and Other Mosquito-Borne Disease Surveillance Working Group, and the Lyme and Other Tick-borne Disease Surveillance Working Group-also bring forward recommendations on zoonotic disease issues for discussion and prioritization to a number of committees and councils in Canada, including:
- Communicable and Infectious Diseases Steering Committee of the PHN
- Council of Chief Medical Officers of Health of Canada
- Council of Chief Veterinary Officers
- PHAC-CFIA Non-Enteric Zoonotic Diseases Steering Committee
- Federal Partners in Wildlife Health Director General Committee
Laboratory capacity for zoonoses and animal health
In Canada, public health and animal health laboratories are often housed together. However, they are not shared laboratories. There are several mechanisms in place for sharing data and information between human and animal laboratories when an urgent issue requires a collaborative approach. Although there are processes in place for sharing information, specimens, data and reports between public health and animal health laboratories as part of regular business, this information is not necessarily shared systematically as livestock laboratory results and human diagnostic test results are usually confidential.
Animal population surveillance systems
The Canadian Food Inspection Agency is the primary federal organization dealing with reportable animal diseases in Canada of significance to human or animal health or to the Canadian economy. The CFIA's surveillance work is supported by the Canadian Animal Health Surveillance Network and the Canadian Animal Health Surveillance System. Both are networks of animal health diagnostic laboratories and surveillance activities created to help integrate animal health surveillance data across multiple jurisdictions.
The Canadian Wildlife Health Cooperative is a node of the Canadian Animal Health Surveillance System. Its regional centres are located in Canada's veterinary colleges and animal health centres. It maintains a centralized national repository for wildlife health data, including diagnostic and testing data, as well as observational data (e.g. citizen science) and other external sources of data.
The Cooperative generates situational awareness and reliable assessments for federal departments on the state of wildlife health in Canada. The Cooperative and the U.S. Geological Survey's National Wildlife Health Center are a joint World Organization for Animal Health collaborating centre, with expertise in various fields of wildlife health and disease.
The Public Health Agency of Canada collects data on the incidence of food-borne illness, including those of animal origin, through its National Enteric Surveillance Program. In collaboration with provincial and territorial partners, PHAC also conducts animal surveillance for non-enteric zoonotic diseases of public health significance. This includes national surveillance of ticks and Lyme disease, surveillance of West Nile virus, and rabies surveillance in livestock and wildlife.
During West Nile virus season, for example, the CFIA shares reports with PHAC on data from domestic animals (such as horses) testing positive for West Nile virus. This data, along with other indicators, allows local and provincial public health officials to assess the current risk to humans from West Nile virus.
PHAC and the CFIA co-fund collaborative surveillance activities with provinces, territories and NGOs for zoonotic diseases, such as avian influenza in waterfowl and shorebirds. In areas with endemic terrestrial rabies, provinces and territories monitor wildlife for rabies through their own laboratories and those of the CFIA. Most provinces have programs to monitor and manage priority zoonoses in animals, for example Quebec rabies control programs (Available in French only), Ontario Rabies in wildlife, New Brunswick Rabies Control Program and Nova Scotia Rabies Response Plan.
Privacy and mandate issues related to sharing data can affect the sharing of surveillance reports between public health and animal health laboratories. Common platforms such as the Canadian Network for Public Health Intelligence support the integration of many surveillance initiatives across human, animal, food, and environment domains.
In the event of a suspected zoonotic outbreak, Canada has two key mechanisms in place to ensure collaboration and information sharing between human and animal health sectors at the federal level: PHAC's Health Portfolio Operations Centre and the CFIA's National Emergency Operations Centre. All groups implicated in the response to an outbreak share reports and intelligence through these emergency operations centres via their supporting infrastructure and incident command systems.
For information on livestock populations, see the World Organization for Animal Health Performance of Veterinary Services Evaluation for Canada. Government departments at all levels, with a mandate for wildlife management and nature protection, monitor wildlife population status and trends in support of conservation goals.
Linkages between human and animal disease surveillance and outbreak response
Human and animal health programs are established in various federal, provincial and territorial government departments and agencies. However, depending on the requirements of the event, ministries of health, agriculture, wildlife, environment, natural resources, conservation and parks do collaborate on zoonotic disease detection, response and control, with the lead organization engaging others as appropriate.
Linkages are also created through a variety of governance structures, such as the Federal Partners in Wildlife Health Director Generals' Steering Committee, which brings together Environment and Climate Change Canada, PHAC, the CFIA and other partners. Committee members collaborate on wildlife health and disease issues; set federal goals and program priorities; and provide federal input to and coordinate funding for the Canadian Wildlife Health Cooperative-a key partner in wildlife surveillance, assessment, investigation and response in Canada.
Canada is developing a National Wildlife Health Strategy that describes the country's vision for wildlife health and identifies the challenges, opportunities and actions involved in achieving that vision. A working group of federal partners is collaborating with the Canadian Wildlife Health Cooperative and provinces and territories on the Strategy.
Canada has a Plant and Animal Health Strategy that sets out a vision for Canada consistent with One Health principles. It describes how multiple organizations will coordinate their activities to safeguard plant and animal health in Canada. Wildlife considerations are included in the strategy primarily in relation to the interface between managed and unmanaged populations.
Public reporting and information sharing
In Canada, situational awareness reports and reports of new, possible and ongoing zoonotic disease threats are routinely shared between agencies through working groups established to manage specific issues, such as avian influenza and chronic wasting disease. These reports are posted on the Canadian Wildlife Health Cooperative website.
More recently, reports on potential new issues and updates on existing issues are shared between agencies through the PHAC-CFIA Non-Enteric Zoonotic Disease Steering Committee. The situational awareness report on the first recorded human case of locally-acquired eastern equine encephalitis in Ontario in late 2016 is an example of inter-agency collaboration for reporting and information sharing.
In early November 2016, the CFIA alerted PHAC to a bovine tuberculosis investigation in Alberta and Saskatchewan. Since then, regular updates on the situation have been shared through the Council of Chief Veterinary Officers calls, bilateral technical exchanges, emergency operations centres, and public affairs communications activities. In addition, PHAC is working with provincial agriculture and health personnel to monitor for possible human exposures or cases.
PHAC and the CFIA have collaborated on assessing the risks associated with animal importation, such as monkeypox in animals from endemic areas of Africa; and on responses to outbreaks of concern to animal health, animal welfare, and human health, such as pandemic influenza pH1N1 in 2009.
P.4.2 Veterinary or animal health workforce
Graduate-level training
There are five veterinary colleges in Canada with active research and teaching programs and that operate veterinary diagnostic laboratories: the Faculty of Veterinary Medicine, University of Calgary; the Ontario Veterinary College, University of Guelph; the Atlantic Veterinary College, University of Prince Edward Island; the Faculté de médecine vétérinaire (Available in French only), Université de Montréal; and the Western College of Veterinary Medicine, University of Saskatchewan.
Canadian veterinary colleges are accredited by the American Veterinary Medical Association Council on Education. Through this accreditation, all veterinarians graduating from accredited Canadian schools are also accredited by the Royal College of Veterinary Surgeons of the United Kingdom.
Veterinary medicine courses in these institutions are based on international best practice and they reference the World Organization of Animal Health "Day 1 graduate" competencies. Veterinarians may become certified in a number of specialties, such as toxicology, veterinary microbiology, veterinary pathology and veterinary preventive medicine (with subspecialties in epidemiology, food safety and veterinary public health).
The minimum professional standard for practicing veterinary medicine in Canada is graduation from a recognized school of veterinary medicine, a certificate of qualification from the National Examining Board of the Canadian Veterinary Medical Association and appropriate provincial or territorial licensure.
Supplemental veterinary public health training opportunities
Veterinarians with degrees in public health and epidemiology at the graduate level are eligible to participate in PHAC's Canadian Field Epidemiology Program. A small number of veterinarians have participated in the program as either students or teachers since the program's inception in 1975.
PHAC and the CFIA are currently working to build Canada's veterinary epidemiology capacity through on-the-job training opportunities, such as hiring veterinary epidemiology students and recent graduates, Agency interchanges, and initiating collaborative projects.
Several provincial ministries of health have engaged veterinary public health epidemiologists in their infectious disease programs, including British Columbia's Centre for Disease Control, Manitoba Health Seniors and Active Living, and the Ontario Ministry of Health and Long-term Care.
P.4.3 Mechanisms for responding to infectious and potential zoonotic diseases are established and functional
National strategies for zoonotic outbreaks
Canada's Public Health Response Plan for Biological Events (2017) covers a range of biological public health events, including zoonotic disease outbreaks. It lays out a scalable governance framework for a coordinated health sector response to large-scale, multi-jurisdictional domestic events and international events, such as pandemic influenza, Zika virus, and Ebola virus, which require national coordination.
Canada also has a number of disease-specific plans and strategies in place, which lay out how various sectors collaborate during a response to a zoonotic disease outbreak, including:
- North American Plan for Animal and Pandemic Influenza
- Canadian Rabies Management Plan, 2009 (currently being updated)
- Federal Framework on Lyme Disease, 2017
- Food-borne Illness Outbreak Response Protocol, 2010 (guides how federal, provincial and territorial public health and food safety authorities manage food-borne illness outbreaks)
Examples of coordination between the sectors
Canada's National Plant and Animal Health Strategy, 2017 was developed in collaboration with industry, NGO, academic and government partners.
Health Canada, the PHAC and the CFIA have a Memorandum of Understanding for Common Issues Related to Human Health (2008). The Agencies have signed a Letter of Agreement Regarding Zoonotic Surveillance and Risk Assessment Process (2008). Recent zoonotic events that required a coordinated response across different sectors and levels of government include:
- Seoul virus (2017)
- Zika virus (2016/17)
- Bovine tuberculosis (2016)
- First recorded human case of locally-acquired eastern equine encephalitis (2016)
- Influenza [H3N2v (2016); H5N2 - low path (2016); H5N2 HPAI (2015); H5N2 HPAI (2014)]
- Ebola virus (2015/2016)
- Salmonella in live chicks (2015)
- Chikungunya (2014)
During these events, and consistent with the scale of the event, information was shared within the Health Portfolio and with other federal, provincial and territorial partners, international stakeholders, and non-government partners. Information sharing was done through regular meetings and calls as part of the emergency operations centre's business cycle for the event and through the responsible program area as appropriate.
During the response to Zika, Canada shared information on numbers of cases with the World Health Organization through the Pan-American Health Organization (PAHO), following International Health Regulation (IHR) procedures. During Ebola, a working group made up of representatives of different sectors produced guidance documents, which were shared on-line, including guidance for veterinarians provided by the Canadian Public Health Association.
During an event, sectors meet regularly but the frequency of meetings depends on the needs of the specific event-the severity, impact and how quickly the situation evolves. Roles and responsibilities for zoonotic disease outbreak response and control depend on the disease under investigation and whether it is reportable or notifiable in animal and human health at the provincial and territorial or federal level. In general, if the disease is not reportable in animals, then the responsibility to investigate and implement public health actions often falls to public health authorities.
During the response to Salmonella in live chicks, local public health authorities handled the human health investigation of cases. Provincial public health authorities coordinated the investigation within each respective province; and PHAC coordinated the investigation overall. The animal health investigation was led by the provincial agriculture authority where the hatchery operated with support from affected provinces.
Provincial public health coordinated the overall investigation into the Seoul virus outbreak (which affected only one province), while local public health conducted the human health investigation of the cases. Provincial agriculture helped develop testing guidelines, public communications, and engaged the veterinary community. PHAC communicated with key international stakeholders, the WHO/PAHO and other IHR National Focal Points and the U.S. Centers for Disease Control and Prevention. The National Microbiology Laboratory, the only laboratory in Canada with the required expertise, provided diagnostic support.
The CFIA led the response to avian influenza and also played a primary role in disease control on affected farms. Local and provincial public health conducted the human health investigations, while PHAC supported the CFIA and provincial public health authorities as needed.
Best practices, challenges, gaps and recommendations
Canada has robust systems in place at all levels of government to prevent, detect and respond to enteric and non-enteric zoonotic disease outbreaks. (For details on enteric zoonoses, see section P5: Food safety.)
There are many well-managed animal and human surveillance systems in place at all levels in Canada to monitor for emerging and re-emerging zoonotic diseases with the potential to affect human populations. Canada can improve its capacity to integrate the animal and human health sectors, particularly when it comes to timely sharing of surveillance data and collaboration on response and control activities.
One Health principles are generally accepted in Canada as key to understanding and managing zoonoses. The integration of human, animal and environmental factors is often considered and applied in policy and program decision making and in the response to rapidly changing disease dynamics at the interface of those factors.
Although Canada does not have a national One Health strategy, One Health ideas have been integrated into several national strategies, including the draft National Wildlife Health Strategy and the National Plant and Animal Health Strategy. Moreover, many public health governance structures and technical or policy working groups that deal with zoonoses follow One Health principles.
Canada has surveillance systems, plans, decision-making algorithms, protocols and partnership agreements that guide and support the response to zoonotic disease outbreaks. The Food-borne Illness Outbreak Response Protocol covers enteric zoonoses, while several disease-specific response plans support outbreaks of non-enteric zoonoses. As well, Canada's Public Health Response Plan for Biological Events could support the response to a large-scale zoonotic disease event.
The spread of infectious disease from animals to humans presents unique challenges that include complex scientific assessments, information sharing across sectors and jurisdictions, and the involvement of diverse groups of experts from multiple cross-sectoral organizations. While Canada has done significant work to build its capacity to respond to zoonotic disease events, there are still steps that could be taken to move closer to a comprehensive One Health approach, including:
- Continue to strengthen the integration of animal and human surveillance and reporting systems.
- Enhance response plans to include clear roles and responsibilities in the coordination, investigation and response to multi-disciplinary, multi-jurisdictional zoonotic disease events.
- Establish informal national working groups to address issues related to priority non-enteric zoonotic diseases.
P5: Food safety
Joint external evaluation target: States parties should have surveillance and response capacity for food and water borne disease risk or events. It requires effective communication and collaboration among the sectors responsible for food safety and safe water and sanitation.
Level of capability in Canada
Canada has a strong food safety system for the prevention and control of food-borne illnesses. This system is implemented through regulatory, public health, and risk analysis efforts across the farm-to-table continuum. Canada's federal food safety system is built on internationally recognized standards and a risk-based inspection approach. Recognized in the 2014 World Ranking Food Safety Performance report, as an overall leader in food safety performance, Canada is consistent in setting best practice standards in these areas.
Government responsibility for food safety and outbreak response is shared among federal, provincial and territorial governments. Canada's ability to anticipate, mitigate and respond to food safety risks requires the cooperation of federal partners, health professionals, local health authorities, and provincial and territorial ministries of health and agriculture.
Government has the authority to establish regulatory standards, inspect facilities, and act to address issues of concern, but industry has the primary responsibility to ensure that food products are safe and meet regulatory requirements.
Canada's capacity to protect the population from unsafe food has grown through improvements to enteric disease surveillance and laboratory diagnostic programs, formal outbreak response protocols and new regulatory approaches. In 2012 the federal government enacted the Safe Food for Canadians Act, which consolidates the authorities of the Fish Inspection Act, the Canada Agricultural Products Act, the Meat Inspection Act, and the food provisions of the Consumer Packaging and Labelling Act. The new act came into force at the same time as the Safe Food for Canadians Regulations and aims to:
- Make food as safe as possible for Canadian families
- Protect consumers by targeting unsafe practices
- Implement tougher penalties for activities that put health and safety at risk
- Provide better control over imports
- Institute a more consistent inspection regime across all food commodities
- Strengthen food traceability
Canada's capacity to detect and respond to food-borne outbreaks has also improved dramatically in the past two decades. Since 1996, the Public Health Agency of Canada (PHAC) has used the PulseNet Canada network of laboratories to "fingerprint" the DNA of bacteria to enhance outbreak detection and define the scope and scale of outbreaks beyond traditional methods.
Following an outbreak of listeriosis in 2008, Canada invested significantly in building its inspection and risk assessment capacity through Renewal of Government Response and Action Plan to the 2008 Listeriosis Outbreak, Food Safety Modernization and Food Safety Oversight.
Indicators
P.5.1 Mechanisms are established and functioning for detecting and responding to food-borne disease and food contamination.
Roles and responsibilities for national food safety and outbreak response
The federal, provincial and territorial governments in Canada share responsibility for food safety and public health. Provincial and territorial public health authorities have jurisdiction under their own authorities for foods produced and sold within the province or territory. The federal government establishes national standards and policies for and addresses issues with multi-jurisdictional and international or trade implications.
Health Canada (Food Directorate) sets standards and policies, which are aligned with the Codex Alimentarius Commission where appropriate, for the safety and nutritional quality of food sold in Canada. Health Canada also:
- Conducts health risk assessments for food-borne hazards
- Manages the safety of veterinary drugs in food-producing animals
- Provides reference services (botulism) and expertise, including expertise in chemical food contamination (for more information on how Canada manages chemical contamination, see section Chemical events below)
Health Canada's Bureau of Microbial Hazards is an International Food Safety Authorities Network (INFOSAN) Focal Point. Health Canada is also responsible for informing Canadians about potential risks to their health.
The Canadian Food Inspection Agency is responsible for:
- Delivering all federal food inspection programs
- Monitoring industry's compliance with food-related acts and regulations
- Conducting food safety investigations (trace back)
- Undertaking enforcement actions, or requesting industry to recall food, as necessary
- Communicating with the public about potential food safety risks
Three groups within the Canadian Food Inspection Agency play key roles in the response to food-borne illness outbreaks:
- Regional inspection staff, including Area Recall Coordinators and Regional Recall Coordinators, conducts food safety inspection activities. Area Recall Coordinators are the usual first point of contact for local or regional health units, provincial and territorial governments, and companies when they identify an issue with their food product.
- The Office of Food Safety and Recall, which coordinates food safety investigations and is responsible for the consistency of decision-making on food recalls. The Office of Food Safety and Recall is the usual first point of contact for national and international food safety related issues and the INFOSAN emergency contact point for Canada. The Office links with Health Canada for health risk assessments and with PHAC for food-borne illness outbreak investigations.
- The Food Safety Science Directorate is responsible for providing scientific analysis and guidance to Canadian Food Inspection Agency staff and provincial and territorial partners.
The Canadian Food Inspection Agency establishes policies and standards for the import of foodstuffs, live animals and animal products, and the Canada Border Services Agency enforces these policies and standards. The Canada Border Services Agency has the legislated power to undertake inspections, seizures, testing, holding, destruction or rejection of products, including live animals, at ports of entry.
The Public Health Agency of Canada plays a leadership role in coordinating the response to multi-jurisdictional food-borne illness outbreaks (outbreaks that occur in more than one province, territory, or country). The Agency also provides support to provinces and territories responding to outbreaks within their jurisdictions when assistance is requested.
PHAC's Infectious Disease Prevention and Control Branch assesses the risk and reduces the impact in Canada and internationally of infectious diseases that can be spread to humans through contaminated food or water, or through contact with infected animals or the environment. The Branch works closely with provincial, territorial and local health departments to conduct ongoing epidemiological disease surveillance for enteric illnesses.
The Branch is the first point of contact for the federal government in managing issues related to food-borne illness outbreaks and an INFOSAN Focal Point. The Branch leads epidemiological and laboratory investigations related to multi-jurisdictional outbreaks. It does this in partnership with Health Canada and the Canadian Food Inspection Agency, and following established protocols. The Branch communicates with Canadians about health risks and how to prevent illness during an outbreak.
PHAC's National Microbiology Laboratory provides reference services for strain identification and characterization, national laboratory-based surveillance, and dissemination of information through PulseNet Canada. This is done through the National Enteric Surveillance Program.
The National Microbiology Laboratory, through PulseNet Canada, is the first point of contact for provinces and territories sharing strain identification data and the detection of clusters of strains that are occurring in more than one jurisdiction-which would identify the potential for multi-jurisdictional food-borne outbreaks. Health Canada provides reference services for botulism, listeriosis and vibrio (in partnership with the National Microbiology Laboratory), as well as reference services for viruses in food.
Canada has a number of mechanisms in place to identify national food safety priorities, including committees within the Public Health Network (described in section P4: Zoonotic disease above). In addition the Federal, Provincial and Territorial Food Safety Committee coordinates the development of national food safety policy options, implements initiatives to achieve national goals, and sets priorities to enhance accountability.
Provinces and territories have their own food safety legislation in place and conduct local epidemiological investigations; inspections of food produced and sold in their jurisdiction; and education activities to reduce risks related to food. They share responsibility with the federal government for inspection of food traded within their jurisdiction. Provincial, territorial and local health departments have the primary statutory authority for enteric disease surveillance in their jurisdiction.
Most food-borne disease outbreaks are local events in just one city and local public health officials investigate. Provincial and territorial health departments have their own outbreak response protocols and may work with their departments of agriculture and the local public health community to guide a coordinated response to food-borne illness outbreaks within their jurisdiction. Provinces and territories may request help from federal departments, such as Health Canada, Public Health Agency of Canada, and the Canadian Food Inspection Agency to respond to an emergency or conduct an investigation.
Industry is responsible for the production of safe food in compliance with all government standards and laws, and for conducting food recalls when required.
Specialized training and resources for food-borne outbreak investigation
PHAC, the Canadian Food Inspection Agency, Health Canada and the provinces and territories have trained resources with experience in food-borne outbreak investigation. These include epidemiologists, microbiologists, medical doctors, veterinarians and other public health professionals (e.g. public health inspectors, public health nurses). At the federal level, all new food inspectors undergo training on their roles and their responsibility to deliver the programs under the Safe Food for Canadians Act. Several PHAC epidemiologists are also trained in food facility investigations and product tracing. In Canada, these trained, experienced people at the national level take part in outbreak response teams.
PHAC's Field Service Training and Response division provides outbreak investigation training through a number of programs, including: Surveillance and Outbreak Core Skills, the Canadian Public Health Service, and the Canadian Field Epidemiology Program.
Food-borne disease outbreak investigations in Canada follow standard epidemiological principles. PHAC is the federal lead for coordinating multi-jurisdictional outbreak investigations, public communications on health risks associated with specific outbreaks, and the steps people can take to protect themselves. The Archived: Food-borne Illness Outbreak Response Protocol guides multi-jurisdictional collaboration in response to food-borne illness outbreaks in Canada by setting out guiding principles and operating procedures to improve the efficiency and effectiveness of response.
PHAC often shares investigation-specific questionnaires for multi-jurisdictional outbreaks with affected provinces and territories to ensure standard data collection and analysis. In some events, investigators will conduct case interviews centrally, using a single interviewer approach. To coordinate a multi-agency response to a food-borne illness outbreak that affects several provinces or territories, Canada establishes an Outbreak Investigation Coordinating Committee with representatives from Health Canada, the Canadian Food Inspection Agency, PHAC and the affected provinces or territories. The Committee assesses information requirements and identifies the partners best suited to gather the required information. Every effort is made to standardize the information collected. Typical investigation steps include:
- PHAC, along with public health staff in the affected provinces and territories and other Committee members, as appropriate, determines what the case definition will be for the investigation.
- PHAC investigators centrally collate and analyze case information (e.g. line lists and case questionnaires) using the information sent to them from provincial and territorial public health partners.
- Findings from the epidemiological, laboratory and food safety investigations are shared with the partner members of the Committee and integrated to identify the potential cause and source of the outbreak and areas for further investigation.
- The local public health units and healthcare oversee collection of appropriate clinical specimens in accordance with infectious disease protocols and laboratory guidelines. The Food-borne Illness Outbreak Response Protocol outlines the standard operating procedure for directing food samples collected during epidemiological, public health, and food safety investigations to the federal laboratory network.
- Led by PHAC's National Microbiology Laboratory, PulseNet Canada is a real-time molecular subtyping network for food-borne disease surveillance supporting the rapid detection and response to single- and multi-jurisdictional outbreaks. PulseNet Canada also provides laboratory investigation during multi-jurisdictional outbreak response and support for single jurisdiction response to enable timely identification of outbreak sources and associated public health action. The work of PulseNet Canada has been critical for the detection and response to large national outbreaks.
Coordination, communication, and collaboration among stakeholders
Federal, provincial and territorial governments work together when there is a national or international outbreak of food-borne illness. They rely on the following tools to ensure effective collaboration:
- The Food-borne Illness Outbreak Response Protocol is the technical and operational protocol that guides how federal, provincial and territorial public health and food safety authorities work together to investigate and manage national or international food-borne illness outbreaks. The Protocol establishes clear lines of communication among all partners and stakeholders and is periodically reviewed and shared with partners and appropriate public health professionals for their endorsement.
- The Multi-Lateral Information Sharing Agreement governs the uses and disclosure of information during public health events and has a specific annex covering Public Health Emergencies of International Concern.
- The interaction between Health Canada and the Canadian Food Inspection Agency during an event or emergency is detailed in a 1999 memorandum of understanding and its related framework, Roles and Responsibilities Framework for Federal Food Safety and Inspection Activities (June 1999).
- A 2008 trilateral memorandum of understanding between Health Canada, PHAC and the Canadian Food Inspection Agency describes the roles and responsibilities regarding common issues that impact human health. These issues include food safety and nutrition, infectious disease outbreak management and emerging zoonotic diseases.
- PHAC, the Canadian Food Inspection Agency and Health Canada have developed the Federal Food Safety Communications Protocol for National Food-Borne Illness Outbreaks (shared in 2015), and a joint protocol for sharing information between Health Portfolio and international partners, including foreign food safety authorities, INFOSAN and the WHO during an event with international implications. (See D3: Reporting)
- The Canadian Food Inspection Agency's Protocol for Sharing of Information during Food Safety Investigations and Recalls describes the type of information that can be shared with other government departments, other countries, regulated parties, third parties and the public during food safety investigations and recalls. It also describes how information is shared and the type of information that the Canadian Food Inspection Agency is obligated to protect. The protocol describes how Canada shares information with other countries to enable follow up action.
PHAC's National Microbiology Laboratory coordinates the collection and centralized analysis of all laboratory data via the PulseNet Canada network. This network is comprised of provincial public health laboratories and federal food safety partners. The National Microbiology Laboratory provides the analyses and interpretations of laboratory data for Outbreak Investigation Coordination Committee discussions.
Federal, provincial and territorial food safety and epidemiology investigators participate in Outbreak Investigation Coordination Committee discussions at which they share information required to mitigate or contain the impact of the outbreak in a timely and effective way. During an event, PHAC, the Canadian Food Inspection Agency and Health Canada have regular conference calls as part of the coordination of information sharing related to new and ongoing events.
The public health and food safety organizations participating in an Outbreak Investigation Coordination Committee each have separate mandates and responsibilities to communicate with stakeholders within their respective portfolios. For example, with respect to routine surveillance, PHAC programs, such as the National Enteric Surveillance Program and FoodNet Canada, routinely communicate with the public health and food safety organizations which represent the farm-to-fork continuum.
The Canadian Food Safety Information Network is a collaborative national (federal/provincial/territorial) network that will strengthen the capacity of food safety authorities across the country to anticipate, detect and respond to food safety events through timely information sharing and coordinated action.
As INFOSAN Focal Points PHAC and Health Canada have close links to international food safety authorities and share information routinely and during emergencies. Canada also has two members on the INFOSAN Advisory Group established in 2006.
PHAC works collaboratively with Health Canada, the Canadian Food Inspection Agency, and with provincial and territorial counterparts to determine risks to the health of the Canadian public associated with food-borne illnesses. The Agency leads public communication on how to prevent food-borne illness during a multi-jurisdictional food-borne illness outbreak. The Agency is also the International Health Regulations (2005) Focal Point (see P2: International Health Regulations coordination, communication and advocacy for more information).
Canada works with several international partners to deal effectively with the international aspect of many food safety issues, and to foster international collaboration. These partners include:
- The U.S. Food and Drug Administration, as part of the Joint Action Plan for the Canada-United States Regulatory Cooperation Council, to develop an agreement to facilitate trade between the two countries based on the assurance that appropriate measures are in place to prevent food contamination and food-borne illness and to detect and respond in the case of an event.
- The U.S. Centers for Disease Control and Prevention and the U.S. Food and Drug Administration to manage and control food-borne illness outbreaks and to enable sharing of laboratory surveillance data in real-time through PulseNet, including whole genome sequence data. PHAC's National Microbiology Laboratory is also a member of the PulseNet International Steering Committee, providing strategic guidance and technical expertise to the international PulseNet network that comprises 86 countries.
- The Asia Pacific Economic Cooperation Food Safety Forum and the Global Food Safety Partnership, to provide technical expertise and to help build global outbreak response capacity.
- WHO (Global Foodborne Infections Network, Foodborne Epidemiology Reference Group, and the Advisory Group on Integrated Surveillance of Antimicrobial Resistance), Pan American Health Organization, Codex Alimentarius, and Food and Agriculture Organization's Working Group to Develop a Handbook for Food Safety Risk Communications. PHAC shares technical and scientific expertise and risk communications advice and has worked on technical papers and guidance documents, such as Applications of Whole Genome Sequencing in food safety management and Guidance for the use of whole genome sequencing in developing countries.
- The Global Health Security Action Group, Communicators Network, with public health and emergency preparedness communicators from the G8 countries plus Mexico, as well as from the WHO and the European Centre for Disease Prevention and Control. PHAC shares communications plans and messages with its international partners, when appropriate, to help foster consistent and coordinated international communications.
- The INFOSAN secretariat, to create and deliver a technical training series for INFOSAN members on food-borne outbreak investigations, food safety investigations, health risk assessment and food recalls, food-borne disease surveillance, and the Canadian Food Inspection Agency protocol for notification of international partners.
Multi-sectoral risk profiling and risk management
Food-borne Illness Outbreak Response Protocol procedures focus on a coordinated response to food-borne illness outbreaks. They do not specifically address the broader risk assessment process that contributes to policy development and standard-setting to reduce the risk of future outbreaks. However, the Protocol's event review process provides an opportunity to capture lessons learned and make recommendations for future policy development to manage risk.
With respect to routine ongoing surveillance, PHAC plays a role in leading surveillance programs that support outbreak detection and providing a more integrated approach to identify and address public health risks in the food chain. The Agency is engaged in cutting-edge science activities, including the development of laboratory technology to enhance the ability to detect and characterize food-borne illness outbreaks and their sources.
PHAC is the lead on the following four ongoing surveillance programs that allow federal, provincial, territorial and local public health authorities to monitor trends, identify potential outbreaks or events, and implement risk management and response strategies:
- The National Enteric Surveillance Program supports timely detection of outbreaks through weekly monitoring of enteric pathogens reported in Canada and provides timely analysis and reporting of laboratory confirmed enteric disease cases in Canada. Program data is used to determine if reductions in food-borne illness are observed over time.
- PulseNet Canada provides high resolution outbreak detection on a daily and weekly basis. Strain-level data on each case of bacterial food-borne illness are analyzed and reported daily and weekly to detect multi-jurisdictional outbreaks. It also provides all data collection analysis during outbreak response.
- The Enhanced National Listeriosis Surveillance Program collects detailed clinical, demographic, and risk factor information on invasive listeriosis cases in participating provinces and territories in order to inform surveillance, research, and outbreak investigations related to invasive listeriosis.
- The FoodNet Canada surveillance program is an integrated food safety surveillance system that protects the health of Canadians by identifying the causes of human illness. By collecting samples from local farms, grocery stores, and water sources and linking this information with human illness, FoodNet Canada can identify the food sources causing illness by region and across Canada. This provides practical public health risk information to the federal, provincial and territorial governments, industry, and national food safety partners. This information is used to enable targeted prevention, prioritize risk mitigation and oversight, and provide evidence on the effectiveness of the food safety system.
PHAC plays a leadership role in public health research along the food continuum to target areas that will improve public health outcomes. Public health research at the molecular and population levels (using methods such as genomics, lab based studies, epidemiological studies, risk modelling, decision analysis, risk mapping and knowledge synthesis) are used to focus on the prevention of public health risks. These risks arise from the food chain, animals and the environment. Information from this research provides the evidence for understanding risks to health and potential control strategies.
It is critical to understand the burden of illness and its associated public health impact and cost, beyond what is captured through laboratory based surveillance. This knowledge is central to measuring the performance of the Canadian food safety system and supporting food safety decision making. Drawing from Canada's multiple complementary food-borne disease surveillance systems, PHAC estimates that each year about one in eight Canadians (four million people) get sick from the food they eat. This accounts for over 11,500 hospitalizations and 240 deaths each year due to food-related illness.
Communication with the public about food safety and food hazards
Provincial and territorial food safety authorities across Canada recognize the importance of effective communications and have dedicated resources in place. Provinces and territories make food safety information, including alerts and advisories, publicly available online. British Columbia's Food Safety & Security site, for example, includes links to food recalls and allergy alerts and public awareness materials, such as "Easy Ways to Make Food Safe." New Brunswick's Food Safety Resources site includes fact sheets, posters and tools, as well as links to federal guidance document and websites.
Canada's Food and Nutrition portal is the public gateway to food safety information from federal government departments and agencies. Some sections of the website allow subscribers to sign up for automatic notifications when information is posted about food recalls and outbreaks.
- Food recalls and alerts
- Canadian Food Inspection Agency food safety investigations
- Public health notices
Authorities that establish Canada's food management systems
The Canadian Food Inspection Agency exercises its food safety mandate under the following authorities. Although not exhaustive, the list illustrates the broad authority of the Canadian Food Inspection Agency and its responsibility in Canada for the whole food continuum:
- Canadian Food Inspection Agency Act
- Food and Drugs Act
- Safe Food for Canadians Act
- Health of Animals Act
- Canada Agricultural Products Act
- Consumer Packaging and Labelling Act
- Feeds Act
- Fertilizers Act
- Fish Inspection Act
- Meat Inspection Act
- Plant Protection Act
- Seeds Act
Health Canada and PHAC exercise their food safety mandates under the Food and Drugs Act, as well as the Department of Health Act and the Public Health Agency of Canada Act, respectively.
Examples of recent food safety actions
In accordance with the Food-borne Illness Outbreak Response Protocol, PHAC leads multi-jurisdictional outbreaks affecting more than one province or territory. Between April 1, 2016 and March 31, 2017, the Agency led nine multi-jurisdictional outbreak investigations-compared to 10 in the previous fiscal year. Descriptions of many of these food-related outbreaks are available on the Agency website, including:
- Outbreak of E. coli infections linked to various flours and flour products, 2017
- Outbreak of norovirus and gastrointestinal illnesses linked to raw and undercooked oysters, 2017
- Outbreak of hepatitis A infections linked to frozen fruit product, 2016
- Outbreak of Listeria infections linked to packaged salad products, 2016
- Archived: Outbreak of Salmonella infections linked to chia and flax seed powder, 2016
- Outbreak of Vibrio parahaemolyticus linked to raw shellfish, 2015
Recent food recalls and investigations include:
- Archived: Salmonella in frozen uncooked breaded chicken, October 2017
- Archived: Marine bio toxin in oysters, October 2017
- Archived: Norovirus in raspberry mousse cake, August 2017
- Archived: Undeclared milk (allergen) in Chinese sausages, September 2017
- Archived: Undeclared gluten (allergen) in infant cereal, September 2017
- Archived: Undeclared sulphites (allergen) in dried and preserved products, August 2017
Reviews and evaluations are essential components of the food safety system in Canada. After a large outbreak or exercise involving multiple stakeholders, the lead agency chairs an "after-action review." This is conducted with appropriate participating partners to produce an after-action report, which informs a capabilities improvement process. For example, following large E. coli events, the Government of Canada developed policy on labelling mechanically tenderized beef, and guidance on E. coli 0157:H7 and E. coli 0157:NM in raw beef.
The Canadian Food Inspection Agency's Program Management Framework is designed to be used proactively and reactively to identify and address food safety risks and performance issues. The Framework was recently used to review and improve performance in the wake of outbreaks associated with E. coli in flour and Salmonella in raw breaded chicken products.
In cases of very serious outbreaks, the Government of Canada will appoint an independent investigator to review the event and make recommendations. For example, following a 2008 Listeria outbreak, the Weatherill Report proposed 57 recommendations to improve Canada's food safety system. The Government of Canada's response to the recommendations was also published online.
Best practices, challenges, gaps and recommendations
Canada has a strong food safety system for the prevention and control of food-borne illnesses. Federal partners work closely with each other as well as with provincial and territorial and local agencies, industry and industry associations, and consumers.
Formalized multi-agency and multidisciplinary participation through every stage of detecting, investigating, and responding to food-borne outbreaks and illness has become routine during multi-jurisdictional outbreak investigations, yet challenges still remain. Multi-jurisdictional outbreaks are difficult to detect and investigate due to the wide distribution and multiple sources of many food ingredients and products.
There are challenges related to timely collection of complete food histories, coordination of specialized laboratory testing to trace and track cases, the viability of viruses and parasites, and unexpectedly contaminated foods sources. Although coordination among federal agencies and experts from health, risk assessment and food sciences is consistent, there is a need to continuously evaluate, modernize, and strengthen the coordination of detection and investigation activities.
Developing new epidemiological tools that enhance food-borne illness outbreak investigations and reduce food-borne illnesses and deaths could help advance food safety capacity. It will be important for the Government of Canada to continue development of next-generation laboratory methods (such as whole genome sequencing) for pathogen identification.
Modernizing the PulseNet Canada network to capture and interpret genomic data is a high priority and is necessary to provide the best outbreak detection and response in Canada and to remain compatible with international partners. PHAC is in the process of transitioning all laboratory-based surveillance to use whole genome sequencing. There is also an opportunity to reinforce coordination and support for provinces and territories by conducting more clinical, food, and environmental testing, and isolate characterization.
P6: Biosafety and biosecurity
Joint external evaluation target: A whole-of-government national biosafety and biosecurity system is in place, ensuring that especially dangerous pathogens are identified, held, secured and monitored in a minimal number of facilities according to best practices; biological risk management training and educational outreach are conducted to promote a shared culture of responsibility, reduce dual use risks, mitigate biological proliferation and deliberate use threats, and ensure safe transfer of biological agents; and country-specific biosafety and biosecurity legislation, laboratory licensing, and pathogen control measures are in place as appropriate.
Level of capability in Canada
Canada has a national biosafety and biosecurity program for the oversight of activities with human and animal pathogens and toxins and regulated plant pests. The ultimate goal of the program is to reduce public health risks and potential risks to Canadian plant and animal resources posed by activities involving these materials.
Biosafety and biosecurity oversight requirements are predominately a federal responsibility, although requirements regarding worker safety, waste and accreditation of diagnostic laboratories exist within governments at the federal, provincial, territorial and municipal levels. Federal oversight has been developed with the objective of complementing existing federal, provincial and territorial regimes to reduce overall burden to regulators and regulated parties.
Indicators
P.6.1 Whole-of-Government biosafety and biosecurity system is in place for human, animal and agriculture facilities
Accountability for dangerous pathogens and toxins and regulated plant pests
Accountability for dangerous pathogens and toxins and regulated plant pests in Canada is a responsibility shared by federal, provincial and territorial partners. At the federal level, accountability is a collaborative effort between multiple departments including the Public Health Agency of Canada (PHAC), the Canadian Food Inspection Agency (CFIA), Global Affairs Canada, Environment and Climate Change Canada, Health Canada, Transport Canada, and the Canada Border Services Agency.
PHAC's Centre for Biosecurity is the national authority on biosafety and biosecurity for human pathogens and toxins and is responsible for their regulation under the authority of the Human Pathogens and Toxins Act and Human Pathogens and Toxins Regulations. PHAC is also responsible for the importation or transfer of terrestrial animal pathogens and toxins, with the exception of non-indigenous animal pathogens, emerging animal disease pathogens, and animal pathogens in animals, animal products, animal by-products, or other organisms, under the authority of the Health of Animals Act and regulations.
CFIA is the national expert on biosafety and biosecurity for foreign animal diseases and emerging animal diseases. It is responsible for regulating the importation or transfer of non-indigenous animal pathogens and emerging animal disease pathogens. This also extends to animals, animal products, and animal by-products that contain a terrestrial animal pathogen.
The Agency acts under the authority of the Health of Animals Act and the Health of Animals Regulations. It is also responsible for transfer and importation of aquatic animal and bee pathogens under these acts, and is Canada's national plant protection organization. It regulates the import and domestic movement of regulated plant pests and invasive plant species under the Plant Protection Act and Plant Protection Regulations, and the Seeds Act and Seeds Regulations.
Canada seeks to fulfil its obligations under the Biological and Toxins Weapon Convention through participation in the Australia Group. The Group is an informal forum that has developed harmonized export controls for human, animal, and plant pathogens and toxins with dual use potential. These controls also apply to dual use manufacturing facilities, equipment, technology, and software, as well as other items that could be used to test or disseminate controlled agents or used for protection against them.
In Canada, export controls have been implemented through Groups 2 and 7 of the Export Control List schedule. Global Affairs Canada administers export controls for strategic goods and technology under the authority of the Export and Import Permits Act. Residents of Canada wishing to export goods or technology listed on the Export Control List must have a Permit to Export issued by Global Affairs Canada.
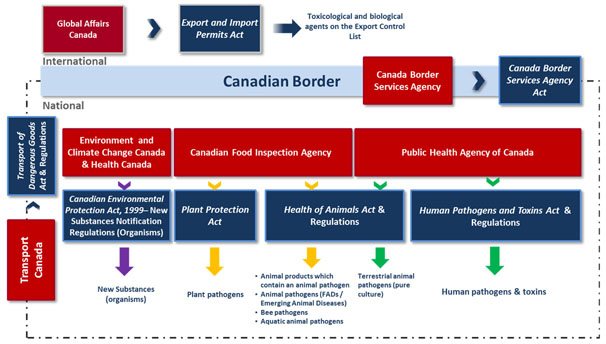
Figure 3: Oversight of pathogens, toxins and regulated plant pests in Canada - Text description
Figure 3 illustrates the oversight of pathogens, toxins and regulated plant pests in Canada and highlights that it is a responsibility shared by a number of departments under the authority of various acts and regulations. Federal departments are depicted with red boxes and the relevant legislation is depicted by dark blue boxes.
Towards the top of the diagram is a long light blue box representing the Canadian border. On top of the box is the word "International". Above it is a box for Global Affairs Canada (GAC) with an arrow pointing to the right towards the Export and Import Permits Act, followed by an arrow pointing to the words "Toxicological and biological agents on the Export Control List", illustrating that GAC manages export controls for strategic goods and technology under this act.
On the Canadian border itself is a box for Canada Border Services Agency (CBSA) with an arrow pointing to the right towards the Canada Border Services Agency Act, indicating that CBSA is responsible for taking action to protect Canada's borders under this legislation, In this case, specifically monitoring the import and export of pathogens, toxins and regulated plant pests for federal partners.
Underneath the Canadian Border box is the word "National" and stemming from either side of the Canadian Border are dotted lines that go down and meet in the middle to form a box. This represents the oversight conducted domestically with respect to biosafety and biosecurity. On the left hand side of the dotted line is a box for Transport Canada, with an arrow pointing to the Transport of Dangerous Goods act and regulations. This describes Transport Canada's cross cutting role with respect to the safe and secure transport of pathogens, toxins and regulated plant pests.
In the centre of the diagram are the remaining departments with a key function in this area. These include Environment and Climate Change Canada (ECCC), Health Canada (HC), Canadian Food Inspection Agency (CFIA), and Public Health Agency of Canada (PHAC).
On the left side is a box representing ECCC and HC, with a purple arrow pointing down to a the Canadian Environmental Protection Act, 1999-New Substances Notification Regulations (Organisms). There is another purple arrow pointing down from there to the words New Substances (Organisms).
In the Centre is a box for CFIA with 2 yellow arrows pointing to 2 boxes. The first box "Plant Protection Act" has a yellow arrow leading to the word "Plant pathogens" and the second box "Health of Animals Act and regulations", has another yellow arrow pointing to the following list:
- Animal products which contain an animal pathogen
- Animal pathogens (Foreign Animal Diseases (FADs)/ Emerging Animal Diseases (EADs))
- Bee pathogens
- Aquatic animal pathogens
This illustrates CFIA's role in biosafety and biosecurity by overseeing laws governing plant pathogens, and some animal pathogens.
The final box on the right is for PHAC with 2 green arrows pointing to 2 boxes. The first box "Health of Animals Act and regulations" has a green arrow leading to the word "Terrestrial animal pathogens (pure culture), and the second box "Human Pathogens and Toxins Act and regulations", has another green arrow pointing to the words "Human pathogens and toxins". This highlights PHAC's responsibility with regards to both human and terrestrial animal pathogens and toxins, working within this legislative framework.
Environment and Climate Change Canada and Health Canada conduct environmental and indirect human health risk assessments, respectively, for new organisms in products regulated under the Food and Drugs Act (e.g., novel foods, human biologics and food additives) and recommend risk management measures.
Certain new living organisms, including microorganisms, proposed for importation into or production within Canada are subject to the Canadian Environmental Protection Act 1999 and the New Substances Notification Regulations (Organisms). A new substance, such as a microorganism developed through biotechnology, requires notification under the Canadian Environmental Protection Act 1999 prior to importation into or manufacture in Canada, if it is not already on the domestic substances list. This legislation protects both the environment and human health from potentially harmful animate biotechnology products.
Substances, including waste, containing pathogens classified as infectious, or toxins identified as toxic in transport are governed by the Transportation of Dangerous Goods Act and regulations, which is administered by Transport Canada.
The Canada Border Services Agency provides integrated border services that support national security and public safety priorities while facilitating the free flow of persons and goods. Under the Customs Act, border service officers have the authority to detain and examine any goods at the Canadian border. The Canada Border Services Agency also provides administrative support at Canadian points of entry for imported and exported pathogens and toxins and plant pests under the authorities of PHAC, the CFIA, and Transport Canada.
Provinces and territories may address other aspects linking to biosafety and biosecurity, including occupational health and safety, waste management and diagnostic accreditation.
National biosafety and biosecurity legislation, regulations, and guidelines
The Human Pathogens and Toxins Act and the Human Pathogens and Toxins Regulations, along with the Canadian Biosafety Standard, 2nd Edition, 2015, and the Canadian Biosafety Handbook, 2nd Edition, 2016, are pillars of Canada's safety and security program for human pathogens and toxins.
The Canadian Biosafety Standard is a harmonized national standard for the handling and storing of human and terrestrial animal pathogens and toxins in Canada. The Canadian Biosafety Handbook specifies the physical containment requirements, operational practice requirements, and performance and verification testing requirements for containment zones where human and terrestrial animal pathogens and toxins are handled or stored. By condition of licence or animal pathogen import permit, the Canada Biosafety Standard establishes the criteria for any containment zone where human or terrestrial animal pathogens or toxins are to be safely handled or stored.
The Canadian Biosafety Handbook is a national guidance document for the safe handling and storing of human and terrestrial animal pathogens and toxins in Canada. A companion document to the Canadian Biosafety Standard, the Canadian Biosafety Handbook provides core information and guidance on how to achieve the biosafety and biosecurity requirements specified in the Canadian Biosafety Standard.
The Canadian Biosafety Handbook addresses the development and maintenance of a comprehensive risk-based biosafety management program. In addition to the Standard and Handbook, Canada has national guidelines for containment level 1 physical design and operational practices, developing a biosecurity plan, and for notifying and reporting under the Human Pathogens and Toxins Act and the Human Pathogens and Toxins Regulations.
Canada's national biosafety and biosecurity regulatory oversight under the act and regulations has the following six components:
- Licensing
- Human Pathogens and Toxins Act security clearances
- Exposure reporting and surveillance
- Pathogen risk assessment
- Learning and knowledge
- Compliance verification, monitoring and enforcement
As specified in section 7(1) of the act, unless otherwise exempted, a licence must be obtained from PHAC to authorize any of the following controlled activities with human pathogens and toxins:
- Possessing, handling or using a human pathogen or toxin
- Producing a human pathogen or toxin
- Storing a human pathogen or toxin
- Permitting any person access to a human pathogen or toxin
- Transferring a human pathogen or toxin to another facility
- Importing or exporting a human pathogen or toxin
- Releasing or otherwise abandoning a human pathogen or toxin
- Disposing of a human pathogen or toxin
Public Health Agency of Canada issues licences under the Human Pathogens and Toxins Act to authorize one or more controlled activities with human pathogens and toxins. The licence specifies which of the controlled activities identified in section 7(1) of the act are authorized and the facility or facilities in which the controlled activities are authorized.
The person to whom the licence has been issued is identified as the "licence holder." The act and regulations detail the specific requirements and obligations of licence holders. The general conditions that apply to every licence are specified in section 4 of the regulations. Additional conditions of licence may also be imposed (Human Pathogens and Toxins Act 18[4]). Compliance with the Canadian Biosafety Standard is a condition of licence.
Biological safety officers keep an updated list of pathogens and toxins that are in the possession of the institution. PHAC keeps a national inventory of which risk groups are held within each facility. For dangerous pathogens and select toxins the exact inventory and location are recorded. Moderate risk pathogen and toxin inventories are maintained and kept at the institutions and reviewed upon inspection or request. The licence holder is responsible for ensuring all persons conducting controlled activities are aware of the licence conditions. A biological safety officer is designated by the licence holder to help implement the licence requirements. The role of the biological safety officer is described in the act (sec 36), regulations (sec 9.1) and Canadian Biosafety Standard (sec 4.1).
The importation into Canada of an animal pathogen or part of one that retains its pathogenicity-or animals, animal products, animal by-products, or other organisms that carry an animal pathogen or part of one that retains its pathogenicity-is regulated by PHAC or the CFIA under the Health of Animals Act and Health of Animals Regulations. Importation of animal pathogens under the act and regulations requires an animal pathogen import permit from PHAC or the CFIA. The act and regulations detail the requirements and obligations of a person handling material imported under an animal pathogen import permit.
The CFIA's Office of Biohazard, Containment and Security under the Health of Animals Act and regulations issues certificates to facilities hosting activities on foreign animal disease and emerging animal disease agents. The biocontainment facilities are inspected, audited, assessed and certified to ensure they meet the physical and operational requirements listed in the Canadian Biosafety Standard, 2nd Edition, 2015. The certificate specifies the pathogens and controlled activities and programs that can be carried out in the authorized facilities.
The Plant Protection Act, Seed Act, and the Canadian Containment Standards for Facilities Handling Plant Pests, 1st Edition, 2007, give the CFIA the authority to prohibit or restrict the movement into, within and out of Canada of any plant pest or other thing that is or could be infested with a pest, or is or could be a biological obstacle to the control of a plant pest.
The Canadian Containment Standards describe the physical and operational requirements for facilities that work with plant pests. The importation into Canada of plant pests, plants or plant products and growing media that carry a plant pest is regulated by the CFIA. Importation and domestic movement of plant pests require a plant pest import permit or a domestic pest movement certificate from the CFIA.
Canada's Export Controls Handbook is a reference tool to assist exporters with questions about Canada's export controls, which are administered pursuant to the Export Control List under the authority of the Export and Import Permits Act. The Handbook contains information on how to obtain the necessary permits for the export or transfer of controlled items and how to comply with the requirements of the Export and Import Permits Act.
Federal outreach
PHAC promotes awareness and conducts inspections to monitor compliance. These activities are based on the premise that the majority of the regulated community will comply with legislative and regulatory requirements if they understand the requirements and have the tools to comply. The federal government publishes laws, regulations and guidelines and engages in outreach and education activities, including:
- Direct communication with stakeholders through consultations, email and the Biosecurity Portal
- Compliance training
- Publication and distribution of Canadian Biosafety Standard, Canadian Biosafety Handbook, and Guidelines
- Distribution of Pathogen Safety Data Sheets
- Biosafety advisories and notifications
Multiple federal regulatory and law enforcement departments and agencies participate in Public Safety Canada's Safeguarding Science initiative to educate academia and the private sector about dual use, security and proliferation risks associated with the use of biological and nuclear materials.
Monitoring activities, inspection and enforcement
PHAC's Compliance and Enforcement Policy outlines its graduated approach to compliance and enforcement. It begins with the least intrusive, such as issuance of letters of non-compliance, and moves progressively to more serious interventions, such as revocation of the licence, seizure, detention and disposal. The CFIA may also use administrative monetary penalties as a regulatory enforcement mechanism.
Compliance is normally achieved through a cooperative approach, but in some cases enforcement actions are needed to correct or prevent non-compliance. Transport Canada also uses a graduated approach and has multiple enforcement tools, such as ticketing for non-compliance, detention notices, notices of direction to remedy non-compliance and notice of direction not to import or to return to place of origin.
PHAC has a law enforcement mandate under the Human Pathogens and Toxins Act and, in some cases, investigates and recommends prosecution to the Public Prosecution Service of Canada. The CFIA and Transport Canada conduct penal enforcement activities respectively under the Health of Animals Act, the Plant Protection Act and the Transportation of Dangerous Goods Act.
The biosafety and biosecurity requirements outlined in the Canadian Biosafety Standard are used by PHAC and CFIA when they monitor regulated facilities and importations of animal pathogens or plant pests as part of the application or renewal process for a licence, permit, or facility certification.
Facility certification is the CFIA's formal acknowledgement that a containment zone or facility
complies with the physical containment, operational practice, and performance and verification testing requirements specified in the Canadian Biosafety Standard or the Canadian Containment Standards. Before issuing an animal pathogen import permit, the CFIA must be satisfied that the activities for which the permit is issued would not result in the pathogen's introduction into Canada or into another country from Canada, or its spread within Canada (Health of Animals Regulations 160[1.1]). Applicants of an animal pathogen or plant pest import permit may be subject to facility certification or compliance verification to demonstrate that the containment zone meets requirements.
For higher risk containment zones, the facility certification process may include an onsite inspection and a review of as-built drawings with specifications. During the commissioning of the facility, it may also include performance and verification testing of critical containment components, the Biosafety Manual, the containment zone standard operating procedures, and, for work with RG4 pathogens, a review of training records.
Facilities certified by the CFIA may require annual recertification to verify ongoing compliance, including a review of documentation, such as program intent and the performance and verification testing reports of critical containment systems.
Inspections of laboratories are an important compliance monitoring activity. PHAC's risk-based approach to selecting which laboratories to inspect and how frequently takes into consideration risk factors, such as compliance history, quality of the facility's biosafety program, category of pathogen (with RG4 inspections every year and RG3 inspections every 3 years), and the complexity of the work done at the facility.
Human Pathogens and Toxins Act and Health of Animals Act inspectors and each facility's biological safety officer are responsible for monitoring compliance at licenced facilities. The function and authority of inspectors is described in the Human Pathogens and Toxins Act sections 40 to 52 and in the CFIA Act subsection 13(3). Biological safety officers have the appropriate knowledge for the containment levels and pathogens and toxins handled at their facility and they support:
- Accuracy and completeness of all licence, animal import and material transfer applications
- Communication with PHAC and the CFIA
- Promotion and monitoring of facility's compliance with legislation, regulations, and standards
- Development and maintenance of biosafety manual and standard operating procedures
- Quality of internal incident investigations
Global Affairs Canada's Trade Controls Bureau is responsible for administering the Export and Import Permits Act. The Canada Border Services Agency and the Royal Canadian Mounted Police enforce it. Where offences are suspected, border services officers may detain or seize goods and forfeiture action may be taken. Corporations and their officers who contravene the Export and Import Permits Act are potentially liable. Investigations can lead to charges, prosecutions, fines and/or incarceration.
The Guidelines for the Notification and Testing of New Substances help individuals responsible for complying with the New Substances Notification Regulations (Organisms) under the Canadian Environmental Protection Act 1999 comply with the rules. Enforcement of New Substances Notification Regulations (Organisms) is the responsibility of Environment and Climate Change Canada enforcement branch.
The Customs Act enables the Canada Border Services Agency to ensure that goods exported from Canada comply with export controls under all acts of Parliament. Border services officers perform compliance monitoring and enforcement at the border.
Laboratory accreditation for biosafety and biosecurity
Although external accreditation is not required under the Human Pathogens and Toxins Act and the Human Pathogens and Toxins Regulations, many laboratories in Canada seek accreditation to comply with other registration bodies or clients. Accreditation can be done through organizations such as provincial colleges of physicians and surgeons, the College of American Pathologists and Accreditation Canada or through compliance with International Organization for Standardization standards.
The accreditation process typically includes rigorous on-site inspections in which auditors examine records, staff qualifications, equipment, facilities, safety records and overall management of the laboratory. Although these processes are primarily focussed on diagnostic quality, there is some overlap with biosafety and biosecurity requirements (see Laboratory audits subsection below).
Funding
As federal programs, all the components of Canada's biosafety and biosecurity activity receive stable and adequate funding through the federal government's annual budgeting process. PHAC reviews its biosafety and biosecurity oversight program every five years to assess value, effectiveness and reach.
Oversight of dual use research
Regulated parties who intend to carry out scientific research under the Human Pathogens and Toxins Regulations must submit, as part of their Human Pathogens and Toxins Act licence application, a plan for administrative oversight of pathogens and toxins in a research setting. The plan eliminates gaps in the oversight of pathogens at the institutional level by establishing effective accountability structures.
Institutions must also ensure that their scientists are aware of potential dual use risks. The Canadian Biosafety Standard (section 2.3.5) describes how to identify dual use risks and PHAC supports awareness through guidelines and training, such as the online Introduction to Dual Use in Live Science Research that encourages responsible conduct of research among scientists, administrators, funding organizations, policy-makers, and the public.
Physical security
All Human Pathogens and Toxins Act licence holders in Canada must have security measures in place to minimize inappropriate removal or release of biological agents. They must also meet physical containment requirements in the engineering and design of their facility. The Canadian Biosafety Standard also requires all Human Pathogens and Toxins Act licence holders and Health of Animals Act permit holders to conduct a biosecurity risk assessment (Canadian Biosafety Standard section 4.1.7) and to develop, implement and evaluate a biosecurity plan to address physical security (Canadian Biosafety Standard section 4.1.11).
Provincial and territorial facilities must participate in the national licencing program. Their governments help licence holders meet those national requirements. For example, Alberta publishes Best Practices for the Assessment and Control of Biological Hazards; Saskatchewan has the Medical Laboratory Quality Assurance Program administered by the College of Physicians and Surgeons to support compliance; and British Columbia is currently developing a comprehensive biosecurity plan for the province. In Quebec, biomedical laboratories must meet the requirements under section 7.1 of the Qmentum Program.
Information security
In Canada access to sensitive information is controlled by a variety of policies and procedures. At the federal level these include:
- Archived: Policy on Government Security, which provides direction on government security to support program and service delivery, and to protect information and individual assets
- Archived: Directive on Departmental Security Management, which supports departments in achieving efficient, effective and accountable management of security
- Archived: Security Organization and Administration Standard, which establishes the standard required by the Security policy (above) and has sections on information and information technology security
- Archived: Operational Security: Management of Information Technology Security Standard, which defines baseline security requirements for federal departments to ensure the security of information and information technology assets under their control.
The Canadian Biosafety Standard includes requirements for restricting access to sensitive information.
Provinces and territories have additional legislation, policies and procedures in place to control access to sensitive information. For example, Alberta's Health Information Act controls access to sensitive health information and Quebec follows standard 24.6 of the Qmentum program addressing information security measures. Finally, the biosecurity risk assessment and plan, which all regulated facilities in Canada must have in place as a condition of their licence, addresses information security (Canadian Biosafety Standard section 4.1.11).
Transportation safety
Transport Canada administers the Transportation of Dangerous Goods Act and Archived: Transportation of Dangerous Goods Regulations, which control the way toxic and infectious substances, including waste, can be imported, transported or shipped. Each province and territory in Canada has adopted the regulations as its own legislation. The act identifies 9 classes of dangerous goods, including toxic and infectious substances (class 6.1 and 6.2 respectively). The regulations include a list of class 6.2 substances by category and describe the shipping requirements for these substances in detail-from containment and labeling to documentation and training.
The Transportation of Dangerous Goods Act (Part 7) requires that before a person handles, offers for transport, transports or imports certain classes of dangerous goods, they must have an approved Emergency Response Assistance Plan in place that describes what is to be done in the event of an accident. Transport Canada verifies and approves these plans under the Act. The National Microbiology Laboratory is the national coordinator for the Emergency Response Assistance Plan program to deal with national emergencies involving human pathogens.
Personnel security and professional qualifications
All licenced facilities in Canada do a biosecurity risk assessment and develop a plan that addresses personnel suitability and reliability. Consequently, they all have procedures for assessing an individual's suitability and reliability. In addition, an HPTA security clearance is required for people who have access to Security Sensitive Biological Agents toxins and pathogens that pose a risk to Canada's national security if misused. The HPTA Security Clearance Program validates that people who have access to Security Sensitive Biological Agents are reliable and trustworthy and do not pose a security risk by virtue of their access.
PHAC provides resources on its e-learning portal to help regulated parties complete their personnel training requirements. For more information on training see the section on Training at laboratory facilities below.
Facility emergency planning
Human Pathogens and Toxins Act licenced facilities have site-specific biosafety and biosecurity management plans in place, such as a biosafety manual, biosecurity plan and standard operating procedures. These materials are informed by risk assessments mandated under the Canadian Biosafety Standard and biological safety officers help develop and maintain them.
Any facility that intends to carry out scientific research must also have a plan in place that sets out administrative measures for managing and controlling biosafety and biosecurity risks in a research setting. The Canadian Biosafety Standard outlines requirements for incidence and emergency response and reporting (Canadian Biosafety Standard 4.9), as well as administrative requirements for records and documentation (Canadian Biosafety Standard 4.10).
Examples include the National Microbiology Laboratory's Incident Response Manual and Biosafety Manual; Alberta Health Services' Code Brown Hazardous Spills Plan and Best Practices for the Assessment and Control of Biological Hazards; and the Northwest Territories' Stanton Territorial Health Authority Biosafety Program Manual. Manitoba's Safety and Risk Management Policy outlines roles and responsibilities in the province related to biosafety and biosecurity management.
Laboratory audits
Laboratories in Canada are audited and/or inspected by PHAC (as national regulator) and/or the CFIA, the facility's biological safety officer, provincial occupational health and safety bodies, and accredited facilities are also audited by external accreditation bodies. Human Pathogens and Toxins Act licenced facilities are required to perform internal inspections and audits at a frequency determined by a local risk assessment.
Some provinces and territories have accreditation or licencing programs for medical/diagnostic laboratories. There are minor differences in the audit systems among provinces and territories. For example, British Columbia has a Mandatory Provincial Laboratory Accreditation Program and Diagnostic Accreditation Program and the provincial laboratory is also accredited through the College of American Pathologists Program. BC's CL2 and CL3 laboratories also have their own internal quality assurance processes.
In Manitoba internal and external safety audits of all laboratory areas are conducted quarterly. Some facilities are audited by external accrediting bodies, while internal audits are conducted by the Workplace Health and Safety Committee or the safety and compliance officer. Manitoba's public health focused laboratory is accredited by the College of American Pathologists. Laboratories in the Northwest Territories are inspected on a daily, monthly and annual basis by hospital staff, following the government's Stanton Territorial Health Authority Biosafety Program Manual.
External audits are conducted by various levels of government or arm's length agencies, for example:
- The laboratory at Stanton Territorial Hospital (Northwest Territories) is accredited through Accreditation Canada.
- Nova Scotia has a program of annual biosafety and biosecurity audits conducted by the provincial laboratory's biological safety officer.
- Laboratories in Quebec (Available in French only) and Prince Edward Island's laboratories are accredited through Accreditation Canada to ISO 15189.
- In Saskatchewan the provincial standards are based on the ISO standard.
- In Ontario, under the Laboratory and Specimen Collection Centre Licensing Act, all clinical laboratories and specimen collection centres must meet the Institute for Quality Management in Healthcare requirements (based mainly on ISO 15189) as a condition of licence. The Institute does assessments as part of accreditation. However, the Ministry of Health and Long-Term Care also has the power to inspect laboratories for compliance with the Laboratory and Specimen Collection Centre Licensing Act.
The National Microbiology Laboratory has accreditation in ISO 9001, ISO 15189, ISO 17025, and ISO 17043 and undergoes internal biosafety and biosecurity audits and external ISO quality audits. Biological safety officers and bio-risk officers do internal audits following the Biological Inventory Assessment Protocol.
The scope of these internal audits may also be assessed by PHAC and the CFIA when they inspect facilities under the HPTA and HAA. Quality, system-based internal audits are performed by trained Quality Office and laboratory personnel as per internal audit procedures.
National laboratories and relevant classifications
PHAC is a designated World Health Organization (WHO) Collaborating Centre for biosafety and biosecurity, while the National Microbiology Laboratory serves as:
- WHO Regional Reference Centre for Measles and Rubella
- WHO National Influenza Centre for Canada
- WHO Collaborating Centre for Emerging and Zoonotic Diseases Detection, Diagnostics, Reference and Research
- PAHO/WHO Polio Regional Reference Laboratory
The CFIA is the Canadian delegate of the World Organisation for Animal Health and its laboratories serve as the World Organisation for Animal Health Collaborating Centre for Food-Borne Zoonotic Parasites and as the Organization's Reference Laboratory for Rabies, Scrapie, Chronic Wasting Disease, Avian Influenza, Classical Swine Fever and Trichinellosis.
Through a number of international partnerships and networks-such as the European Commission, the Global Health Security Action Group Laboratory Network, and the Biosafety Level 4 Zoonotic Network-the National Microbiology Laboratory has established relationships for sharing information and best practices to respond to threats of emerging disease and to monitor global activities.
Reducing access to dangerous pathogens and toxins
The Human Pathogens and Toxins Act and the Human Pathogens and Toxins Regulations do not require dangerous pathogens and toxins to be consolidated in a minimum number of facilities in Canada. Nonetheless, some dangerous pathogens have been consolidated to a minimum number of facilities, for example all RG4 pathogens are consolidated in one jointly operated federal facility in Canada and efforts are underway to reduce the number of facilities in Canada that contain polio virus.
In addition, many Canadian laboratories use diagnostic tests that eliminate the need to culture dangerous pathogens. The National Microbiology Laboratory minimizes risks as much as possible when developing or selecting diagnostic methodologies and many of its programs have shifted to molecular technologies. However, confirmatory assays are still culture-based.
P.6.2 Biosafety and biosecurity training or plans are in place
Training at laboratory facilities
There are training programs in place at all Human Pathogens and Toxins Act licenced facilities in Canada. Biological safety officers are responsible for arranging appropriate training on applicable biosafety and biosecurity laws, regulations and standards at their facilities. Furthermore, the Canadian Biosafety Standard (section 4.3) outlines training program requirements, which are applicable to the Human Pathogens and Toxins Act, the Human Pathogens and Toxins Regulations, the Health of Animals Act and the Health of Animals Regulations.
PHAC provides resources to help laboratories meet training requirements. The Laboratory Biosafety and Biosecurity e-learning portal gives licence holders and stakeholders access to common course material and instructional videos. Although not mandatory, the training material supports a common curriculum for biosafety and biosecurity in Canada.
The current training requirements and course materials are based on a needs assessment conducted in 2012. The Agency, an accredited American National Standards Institute and International Association for Continuing Education and Training biosafety training provider, is working to align its training modules with the American National Standards Institute's International Association for Continuing Education and Training Standard, which is already reflected in the Canadian Biosafety Standard (sec 4.3.9-10).
The National Microbiology Laboratory provides mandatory biosafety and biosecurity training or refresher training based on a common curriculum at all its sites. It maintains a database of staff training results and conducts annual emergency drills to test staff on procedures.
The Transportation of Dangerous Goods Regulations require that any person who handles, offers for transport or transports dangerous goods be adequately trained and hold a training certificate. With few exemptions, carriers who ship, store or handle infectious substances in Canada have appropriate training. Transport Canada refers carriers who require training to private sector companies that provide appropriate training on the Transportation of Dangerous Goods Regulations. The Department is currently standardizing training requirements and updating them to better reflect current industry practices.
Provinces and territories employ a variety of strategies to meet the staff training requirements of their licences. For example, mandatory biosafety and biosecurity training is part of new employee orientation in Manitoba; Alberta has a provincial competency and acknowledgement program; and the Northwest Territories does annual employee training needs assessments. In Ontario, each laboratory and specimen collection centre must provide Institute for Quality Management in Healthcare induction and refresher training on biosafety and biosecurity.
Oversight of the training and exercise requirement
Public Health Agency of Canada and CFIA inspectors review training documentation to determine whether or not a facility is meeting its training requirements and make recommendations for corrective action, if necessary. At the National Microbiology Laboratory, drills and exercises are assessed through a "hotwash" process that includes gathering suggestions for improvements and drafting an action plan to implement corrections.
Funding for training
PHAC's Biosafety and Biosecurity Training Program has adequate, stable funding for the current task of creating and distributing national bilingual biosafety and biosecurity training resources online. Each licenced facility is responsible for finding appropriate internal training programs that allow them to meet their particular licence requirements.
Context: Facilities and biosafety equipment maintenance
Maintenance planning for new facilities
As part of the Human Pathogens and Toxins Act licencing and the Health of Animals Act permit process in Canada, all new facilities where people work with dangerous pathogens are planned with long-term resource commitments, and all high-risk facilities are formally commissioned before opening.
Availability of medical maintenance and waste management
Under the Canadian Biosafety Standard (section 4.8), decontamination and waste management procedures must be documented in standard operating procedures that are easily understood and implemented by all personnel. The Canadian Biosafety Standard (section 5.1.5) also requires that class II biological safety cabinets be certified in accordance with National Sanitation Foundation International and American National Standards Institute 49 Biosafety cabinetry design, construction, performance, and field certification.
Licenced facilities in provinces and territories have appropriate waste management policies in place and well-documented procedures. For example, Alberta's Waste Management Policy and IPC Community-Based Services Resources Manual: Waste and Sharps Handling; and the Northwest Territories' Stanton Territorial Health Authority Biosafety Program Manual (Appendix 36). In all provinces and territories, except Prince Edward Island and the Northwest Territories, biosafety cabinets can be serviced locally. HEPA Atlantic, located in Nova Scotia, certifies and maintains Prince Edward Island's biological safety cabinets and the Northwest Territories has a training program in place to build local capacity.
Human Pathogens and Toxins Act licence holders and Health of Animals Act permit holders are responsible for maintaining their facilities as specified in the provisions of the Canadian Biosafety Standard. Funding for facility and equipment maintenance varies from province to province-most have adequate funding set aside for timely maintenance but some facilities report that they need additional funding to keep equipment current (Manitoba and Northwest Territories).
Transportation of biological material
Canada's Transportation of Dangerous Goods Regulations covers category A and B infectious substances and is based on the United Nations' Recommendations on the Transport of Dangerous Goods Model Regulations. The Transportation of Dangerous Goods Regulations is updated regularly to harmonize Canadian regulations with UN recommendations. Transport Canada and its provincial and territorial partners enforce the regulations as set out in existing memoranda of agreement between the federal government and each province and territory. The regulations apply to dangerous goods being imported, handled, offered for transport, or transported by air, marine, rail and road modes of transportation.
The Canadian Biosafety Standard sets out requirements for movement and transport of pathogens and toxins within laboratories, and between laboratories located inside the same building as these movements are not included in the scope of the Transportation of Dangerous Goods Regulations (Canadian Biosafety Standard requirements 4.6.21 and 4.8.8).
In provinces and territories, carriers must follow both the national regulations and any relevant provincial or territorial regulations, such as Alberta's Dangerous Goods Transportation and Handling Regulation and Transportation of Infectious Substances guide.
Occupational health and safety
Workplace safety, including safety in laboratories, is generally a matter of federal, provincial and territorial jurisdiction. Provinces and territories are responsible for occupational health and safety and have employer Workplace Hazardous Materials Information System (WHMIS) requirements within their respective jurisdictions. Provincial and territorial occupational health and safety agencies appoint inspectors under their legislation to verify compliance and take measures to confirm work is being carried out safely.
Laboratory personnel in federal and most provincial and territorial laboratories have equal access to occupational health and safety services. Personal protective equipment is readily available in all facilities and immunization policies and standards for laboratory workers (as health care workers) are in place in most provinces and territories. For example:
- Alberta Health Services has an immunization policy and standard for health care workers.
- New Brunswick has a vaccination policy for laboratory personnel.
- Northwest Territories Stanton Territorial Health Authority Biosafety Program Manual covers vaccinations for laboratory personnel.
- Quebec has a guide (Available in French only).
- Newfoundland and Labrador has a provincial immunization policy manual.
- Prince Edward Island has the Recommended Immunization Schedule for Health Care Workers.
In Ontario, the Occupational Health and Safety Act and the accreditation program for laboratory and specimen collection centres promote occupational health services.
Post-exposure prophylaxis is provided to health care workers, including laboratory workers, in facilities across Canada. Most laboratories have post-exposure prophylaxis guidelines, protocols, or plans, such as:
- Alberta's guidelines for non-occupational, occupational and Mandatory Testing and Disclosure Act post exposure management and prophylaxis
- Prince Edward Island's Guidelines for the management of a percutaneous or sexual exposure to blood borne Pathogens
- Quebec has developed the Guide pour la prophylaxie et le suivi après une exposition au VIH, au VHB et au VHC (Available in French only)
The Human Pathogens and Toxins Act (sections 12,13 and 14) and the Canadian Biosafety Standard (section 4.1) set out notification and reporting requirements for licence holders, biological safety officers, and persons conducting controlled activities authorized under a licence. To facilitate notification to PHAC, an on-line Biosecurity Portal (LINC) is available for secure creation, storage and submission of laboratory incident reports and other notifications. Mandatory notification to the Agency is required without delay when an incident involving a human pathogen or toxin has caused, or may have caused, disease in an individual.
Best practices, challenges, gaps and recommendations
Canada's current biosafety and biosecurity oversight framework is very comprehensive and risk based. It covers all sectors working with risk group 2, 3 and 4 pathogens and selected toxins, and its controls are commensurate with the risks. A performance based approach provides multiple means to achieve the same objective, which is the safe and secure use of pathogens and toxins. Increased institutional accountability fosters a culture of risk management within regulated institutions.
From a federal perspective, it is also continually improved through the "life cycle" approach to regulation making articulated in the Cabinet Directive on Regulatory Management. The life cycle approach recognizes that attention must be given not only to regulatory development and analysis but also to the implementation, evaluation, and review of regulations. The life cycle approach improves the effectiveness, efficiency, and accountability of the regulatory system to support the government's commitment to Canadians. A further consultative approach is used to promote policy interventions that are sustainable within the regulated community.
The Government of Canada recognizes that the oversight framework could be enhanced. Canada aims to strengthen biosafety and biosecurity practices through specific deliverables including:
- Optimizing delivery of the biosecurity program
- Addressing potential regulatory duplication among federal authorities
- Recommending action for collaboration between federal partners
- Exploring innovative means to further educate regulated parties
Canada recognizes the challenge of emerging technologies and the need for an oversight framework on dual use in life sciences research and is currently increasing coordination with stakeholders in the other federal departments, provincial and territorial governments, industry, education, research, and the public in order to strengthen an integrated oversight framework that raises awareness of the impact of dual use research at various steps within the research continuum.
P7: Immunization
Joint external evaluation target: A functioning national vaccine delivery system-with nationwide reach, effective distributions, access for marginalized populations, adequate cold chain, and ongoing quality control-that is able to respond to new disease threats.
Level of capability in Canada
In Canada, vaccination is a responsibility shared among the federal, provincial and territorial governments. Provinces and territories deliver the vaccination program, which includes selecting and funding vaccines for their populations, determining their vaccination schedules, designing and delivering immunization programs and monitoring vaccine uptake within their populations. It is important to note that vaccines are not covered by the Canada Health Act and are therefore provided as supplementary services at the discretion of each province and territory.
The federal government has a role in regulatory approval, vaccine safety, bulk purchasing of vaccines, national surveillance for vaccine-preventable diseases and adverse events following vaccination. It also assumes responsibility for national leadership and coordination in knowledge development, innovation, and sharing best practices.
The National Immunization Strategy, established in 2003 by federal, provincial and territorial Deputy Ministers of Health, provides a framework for effective inter-jurisdictional collaboration that improves the relevance, effectiveness, and efficiency of immunization programing across Canada. At the time of its creation, the National Immunization Strategy was designed to address a number of immunization challenges in Canada.
Work under the National Immunization Strategy by all jurisdictions continues through national engagement and collaboration under the Public Health Network Council structure, particularly through the Canadian Immunization Committee and the Vaccine Supply Working Group. The result is that jurisdictions today are better connected, helping ensure that immunization program design and delivery is evidence-based and sustained by a safe and secure vaccine supply chain, which benefits all Canadians.
Canada's updated National Immunization Strategy Objectives 2016-2021 aligns with the World Health Organization (WHO) Global Vaccine Action Plan's principles (ownership, partnership, equity, integration, sustainability and innovation) and strategic objectives.
Indicators
P.7.1 Vaccine coverage (measles) as part of national program.
Current Canadian programs supporting vaccination (in general)
In Canada, responsibility for health care, including immunization, is shared by the federal, provincial and territorial governments. While each jurisdiction has a distinct mandate and unique operating context, their activities are complementary and collaborative.
Vaccine authorization
Health Canada conducts rigorous scientific review and testing of vaccines to assess their quality, safety and efficacy before they are approved for use in Canada. Once a vaccine is licenced for use in Canada, vaccine safety is monitored to detect and respond to adverse events following immunization. Under the Food and Drugs Act and regulations, vaccine manufacturers are required to report to Health Canada any serious adverse events of which they become aware. Most provinces also require vaccination providers to report serious adverse events to provincial public health authorities.
Vaccine recommendations
Recommendations on the use of vaccines in Canada are provided by the Public Health Agency of Canada, and developed through its external advisory body, the National Advisory Committee on Immunization. This group of experts provides transparent technical and scientific analysis and recommendations on new and existing vaccines. Provinces and territories use these vaccination recommendations to assist in the planning of their vaccination programs.
The Committee to Advise on Tropical Medicine and Travel is an expert advisory body that assists the Public Health Agency of Canada with travel health-related advice for travellers and health care professionals. Country-specific vaccine recommendations, along with other related resources, are publicly available on the Government of Canada's travel health website.
Vaccine procurement
The federal government aims to enhance affordability of the vaccine supply in Canada by facilitating provincial and territorial participation in the Bulk Procurement Program to benefit from multi-year contracts with multiple providers. Under the Program, smaller jurisdictions can purchase vaccines at a lower cost than if purchased independently.
Vaccination programs
Canadian provinces and territories implement vaccination programs that align with the goals of their respective health care plans. They take into consideration recommendations, scientific evidence, local epidemiology of diseases, budgets, and public health capacity. They all have programs that provide publicly-funded, universal access to vaccines for infants, children and adolescents. These include vaccines against diphtheria, pertussis, tetanus, polio, H. influenzae type B, hepatitis B, measles, mumps, rubella, varicella, invasive meningococcal disease, invasive pneumococcal disease, and human papillomavirus.
Publicly-funded adult vaccination programs provide universal access to vaccines for tetanus and diphtheria (Td), influenza for pregnant women, adults with chronic medical conditions, and adults 60 or 65 years and older, as well as pneumococcal polysaccharide vaccine for seniors aged 65 years and older.
Indicators
National vaccination coverage goals were developed for childhood, adolescent and adult vaccines that are publicly funded in all provinces and territories. The goals are based on best practices, international standards, and a review of existing data and surveillance systems to set baseline metrics. These include a vaccination goal of 95% coverage for childhood vaccines, 90% coverage for adolescent vaccines, and specific goals for older adults and healthcare workers for relevant vaccines.
Monitoring national vaccination coverage (in general)
The Public Health Agency of Canada conducts national population surveys to report on national vaccine uptake for children and adults, and for seasonal influenza. To gain a clearer understanding of the factors influencing decisions on immunization, the surveys include questions on parental knowledge, attitudes and beliefs, as well as vaccine hesitancy and barriers to immunization.
National immunization coverage estimates provide information on the level of protection against disease in Canada and identify general trends over time in uptake and attitudes toward vaccines. Survey results are used to measure progress towards Canada's national vaccination goals and to provide estimates of coverage to the World Health Organization and the Pan American Health Organization.
In 2015, Canada's vaccination coverage estimates by two years of age were:
- 89% for measles (one dose)
- 91% for diphtheria, pertussis, tetanus and polio (3 doses)
Provinces and territories monitor vaccine coverage using data from different sources, including provincial immunization registries or repositories (if available), day care or school entry records, or results of provincial vaccination coverage surveys (table 3).
Systems for identifying and addressing disparities
Analysis of the 2013 Childhood National Immunization Coverage Survey indicated some inequalities in vaccine uptake. Children of low socio-economic status were at higher risk of being never vaccinated or having incomplete vaccination. The results also indicate a strong association between parents' lack of confidence in vaccine effectiveness and safety and incomplete immunization. In contrast, analysis of data related to human papillomavirus vaccination indicates that higher parental education level has been associated with refusal of the vaccine.
The socio-demographic data and sample size of the national immunization coverage surveys only allow for limited analysis of the determinants of non-vaccination, under-vaccination and barriers to vaccination. National coverage assessment methods did not:
- Identify under and non-vaccinated sub-population groups at the community level
- Describe the extent of under and non-vaccination among various sub-populations, including some potentially vulnerable sub-populations, such as recent newcomers to Canada, Indigenous peoples
- Determine the systemic barriers negatively impacting vaccination access and uptake
Initiatives to address these identified gaps are being developed and implemented under the Government of Archived: Canada's 2016 Budget, in which the Government of Canada committed "to improve Canada's ability to identify under- and un-immunized Canadians and develop a focused program to improve vaccine access and uptake," and through the National Immunization Strategy Objectives 2016-2021 (described in Best practices, challenges, gaps and recommendations).
At the local level, data from immunization registries and school programs have been used to identify neighbourhood immunization rates and disparities. For example, this approach was successfully implemented by the Saskatoon Health Region to identify disparities and reallocate resources to improve vaccine coverage for the entire population.Footnote 3
P.7.2 National vaccine access and delivery
Appropriate management of vaccine stock
The federal government through Public Services and Procurement Canada operates a bulk procurement program for vaccines. The Public Services and Procurement Canada program, which contracts with multiple suppliers for a given vaccine, allows Canada to manage vaccine shortages quickly and ensure equitable, affordable access for all jurisdictions. Approximately 85% of vaccines for publicly funded vaccination programs in Canada are purchased through the program. Provinces and territories that decide to join the program determine their own requirements and pay for the vaccines they use.
The Public Health Agency of Canada co-chairs the Canadian Immunization Committee's Vaccine Supply Working Group, a multi-jurisdictional group that monitors vaccine supply and prices and develops principles, strategies and guidelines to address vaccine supply issues (shortages, quality) and to facilitate fair and equitable distribution of vaccines during a shortage.
In addition to the federal government mechanisms to ensure sustainable supplies of vaccines (mentioned above), provinces and territories rely on best practices, such as good forecasting, inventory monitoring, central oversight, and safety stocks at provincial depots to maintain supply of vaccines.
Vaccine warehouses in Canada must have a Drug Establishment Licence and Good Manufacturing Practice certification from Health Canada. Federal inspectors ensure that warehouses maintain standards for cold chain storage and distribution. Vaccine contracts under the bulk procurement program for vaccines describe the requirements for cold chain maintenance. The supplier maintains the cold chain during delivery to provincial or territorial depots across the country.
Once the vaccine is accepted at a depot, the province or territory is responsible for maintenance of the cold chain during storage and further distribution of the vaccine. The Public Health Agency of Canada issued Archived: National Vaccine Storage and Handling Guidelines for Immunization Providers in 2015. Provinces and territories have policies and issue guidance on cold chain management for public health and health professionals involved in vaccine delivery.
Distribution of vaccine
Although vaccination is a personal choice in Canada, it is strongly encouraged by federal, provincial and territorial authorities. The federal government supports education and promotion campaigns to encourage vaccination, while provinces and territories use a variety of strategies from education and vaccine awareness campaigns to regulations that require reporting vaccination status for school or day care entry.
Vaccination delivery and information systems in Canada
Most provinces and territories offer information about vaccines on their websites and make vaccines available through a network of health care providers (public health nurses, pharmacists, physicians) in a variety of settings. Vaccination delivery models vary across Canada (Figure 4). British Columbia, Manitoba, Ontario, Quebec, New Brunswick, Nunavut, and Nova Scotia have mixed models involving primary health care providers and public health clinics. Prince Edward Island, Newfoundland and Labrador, Saskatchewan, Alberta, the Northwest Territories and Yukon Territory rely mainly on public health for vaccination delivery.
All provinces and territories deliver school-based vaccination programs (where nurses vaccinate students in temporary clinics set up in schools) for vaccines recommended for school-age children and youth (i.e. hepatitis B vaccine and the human papilloma virus vaccine). This approach optimizes access to vaccines for children and youth.
Pharmacists' scope of practice has expanded in recent years, with vaccine injection authority in all provinces and territories except Quebec, Yukon, Nunavut, and the Northwest Territories. The minimum age for clients to be eligible for pharmacist administered vaccine varies by jurisdiction. Pharmacists play an important role in the delivery of the seasonal influenza vaccine, accounting for >50% of doses given in some jurisdictions.
Depending on the vaccination delivery system, received vaccinations are recorded in the child's medical record, parent-held record, administrative data, school board record and, when available, in a provincial or territorial immunization information system. The type of vaccination delivery program influences the completeness of information found in these records.
Figure 4: Immunization delivery and information systems in Canada - Text description
This figure uses a map of Canada to describe the immunization registry landscape in Canada and looks at both immunization delivery and information systems used across the country. Vaccination delivery models vary across Canada. The map indicates that British Columbia, Manitoba, Ontario, Quebec, New Brunswick, Nunavut, and Nova Scotia have mixed models involving primary health care providers and public health clinics. The map highlights that Prince Edward Island, Newfoundland and Labrador, Saskatchewan, Alberta, the Northwest Territories and Yukon Territory rely mainly on public health for vaccination delivery.
The majority of provinces and territories (12 of 13) are in the process of implementing electronic immunization registries. The map indicates that Yukon Territory, British Columbia, Saskatchewan, Manitoba, Ontario, Quebec and Nova Scotia all use Panorama. The remaining jurisdictions use other registries or repositories. The map shows that: Alberta uses Imm/ARI; Northwest Territories uses a Manual database; Newfoundland and Labrador uses CRMS; New Brunswick uses CSDS+3; PEI uses ISM and Nunavut does not employ an electronic registry or repository at this time.
Immunization registries have the capacity to quickly and accurately capture data on the administration of vaccine doses at the individual level and are an important tool in determining the level of protection in a population against vaccine-preventable diseases. The majority of provinces and territories (12 of 13) are in the process of implementing electronic immunization registries, however these are not yet fully functional and do not have the ability to report coverage rates on the entire population.
Provinces and territories are using various strategies to improve the completeness of records in their immunization registries. These include:
- Providing web portal access to the public and health professionals
- Working with electronic medical record vendors to enhance interoperability between electronic medical records and registries
- Enacting legislation to make it mandatory for health care professionals to enter immunizations directly into the registry system
Immunization programs led by the Government of Canada
First Nations living on reserve
The federal government provides primary care and public health services to First Nations living on reserve through policies to improve access to services. This includes providing for immunization services and delivering provincially purchased vaccines according to provincial schedules. Health Canada works with the local public health unit to ensure cold chain integrity during the transportation of vaccines from provincial depots to First Nations communities through the application of provincial standards and guidelines.
Health Canada either directly delivers, or financially supports First Nations communities and/or organizations in the delivery of culturally appropriate immunization programming in First Nations on-reserve communities in Alberta, Saskatchewan, Manitoba, Ontario, Quebec and the four Atlantic provinces. Health Canada's immunization programs work to ensure that immunization services on-reserve align with programming delivered by provincial governments to the general population. It produces culturally relevant education and awareness products targeted to First Nations on-reserve and supports education and awareness activities for Inuit populations in collaboration with its national Inuit partner, Inuit Tapiriit Kanatami.
Health Canada also monitors immunization coverage among First Nations living on reserve to measure progress against national vaccination goals, as well as to identify disparities and to inform program interventions.
Vaccinations are reported through aggregate community-based reporting on an annual basis. Coverage information is further summarized at the regional and national levels. Overall coverage estimates are produced for vaccines where there is limited variation between provincial vaccine schedules. First Nations living on reserve may visit off reserve primary care services for vaccinations. Vaccines received off reserve may not be recorded in community records, which may lead to an under-estimation of coverage estimates for First Nations who live on reserve.
In British Columbia, the First Nations Health Authority has responsibility for health service design and delivery, including immunization programming for First Nations living on reserve. First Nations populations living off reserve and Inuit receive immunization services from the province and territory and Inuit regions in which they reside
Canadian Forces
The Canadian Armed Forces maintains its own Immunization Standard, based on existing national guidance, under which it provides routine vaccinations to service members as well as travel or operationally indicated vaccines (such as rabies, typhoid, yellow fever). The Archived: Canadian Forces Health Information System is used to electronically record all everyday management and delivery of health and dental services, including immunizations.
The Directorate Force Health Protection also collects statistics from individual Canadian Forces clinics on influenza vaccination rates (and specifically immunization of uniformed health care and dental workers). The Department of National Defense participates in Canada's Vaccine Supply Working Group (described previously). In terms of program delivery, Canadian Forces Bases are staffed with dedicated immunization nurses, who deliver vaccination services.
Immigration, Refugees and Citizenship Canada
The Government of Canada's Interim Federal Health Program provides limited, temporary coverage of health-care benefits in Canada to resettled refugees, asylum seekers, and certain other groups, until they become eligible for provincial or territorial health-care coverage. The Program provides basic coverage (i.e. hospital, physician) similar to provincial or territorial health-care insurance, as well as supplemental services (i.e. vision, urgent dental, counselling) and prescription drugs, including vaccinations, similar to provincial vaccine schedules.
Starting April 1, 2017, the Interim Federal Health Program was expanded to cover certain services for individuals who have been identified for resettlement before they come to Canada. These pre-departure medical services include coverage of certain pre-departure vaccinations aligned with Canadian guidelines. These vaccinations are not mandatory for immigration to Canada; they are only given to individuals who consent.
As well, pre-departure medical services include services to manage communicable disease outbreaks in refugee camps, such as measles or influenza. It is expected that these services will prevent the introduction and spread of vaccine-preventable diseases into Canada, and reduce morbidity and mortality among refugees. In addition, the Program will help maintain measles, rubella and congenital rubella syndrome elimination status in the WHO Americas region; support the WHO Global Polio Eradication Initiative; and facilitate rapid school entry for displaced children.
Ensuring the capability for rapid distribution in an emergency
Canada's pandemic vaccine strategy aims to:
- Provide a safe and effective vaccine for all Canadians as quickly as possible
- Allocate, distribute, and administer vaccine as efficiently, equitably and effectively as possible
- Monitor the safety and effectiveness of pandemic vaccine
In addition to its usual role providing regulatory authorization for vaccines, the federal government also negotiates and establishes ongoing contracts for pandemic vaccine. In the event of a pandemic, Canada would leverage its existing vaccine distribution networks as it did during the H1N1 pandemic, 2009-2010.
Health Canada works to support on-reserve First Nations communities in building, strengthening and testing communicable disease emergency plans that include pandemic influenza response. Health Canada also works with the Public Health Agency of Canada and other federal partners, national and regional First Nations organizations, and provinces to ensure comprehensive and coordinated communicable disease emergency planning and response activities in on-reserve First Nations communities.
Best practices, challenges, gaps and recommendations
While immunization coverage in Canada today is good and progress is being made toward common objectives, more is needed in Canada to reach national vaccination coverage goals. Furthermore, while rates of vaccine-preventable diseases in Canada are low, recent measles and pertussis outbreaks demonstrate that Canadians are still at risk.
In its 2016 Budget, the Government of Canada committed $25M over five years to increase immunization coverage rates. In addition, federal, provincial and territorial governments have worked together to establish a five-year set of objectives for the National Immunization Strategy that can capitalize on this new federal investment, leverage momentum and provide focus to their collective efforts.
The National Immunization Strategy Objectives 2016-2021 include:
- Setting national vaccination coverage goals and vaccine-preventable disease reduction targets
- Understanding barriers to vaccine uptake in under-immunized populations
- Ensuring timely and equitable access to vaccination services
- Developing evidence to inform effective program planning, with a view to increasing vaccination coverage
Goals for vaccination coverage and vaccine-preventable diseases reduction targets have been endorsed by provincial, territorial and federal governments via the Public Health Network Council. Progress towards reaching the national vaccination coverage goals and vaccine-preventable disease reduction targets will be reported every two years, using national coverage surveys and vaccine-preventable disease surveillance systems.
- Canadians now have access to an online and mobile app to keep track of their family's vaccinations with the CANImmunize mobile app. CANImmunize is a free, bilingual tool that is available nationwide.
- Methods used for national vaccination coverage surveys have been revised to measure vaccine hesitancy, barriers to vaccination, and to include First Nations (living off reserve), Inuit, and Metis children under two years of age.
- The mandate of the National Advisory Committee on Immunization includes scientific and economic evaluation, which will help to make vaccination program decision-making faster.
- The Canadian Institutes for Health Research in partnership with the Public Health Agency of Canada launched a funding opportunity for research to identify under- and non-immunized populations, and the socio-structural barriers associated with lower immunization access and uptake, in the Canadian population, with a particular focus on childhood immunization.
- The Canadian Immunization Research Network supports many research projects on vaccination issues. The Network will receive $10 million between 2017 and 2021. Of this, $2 million will be used for research on vaccination acceptance and uptake.
- A new Immunization Partnership Fund was launched to support provinces and territories and other partners in carrying out projects that improve vaccination access and uptake by identifying key barriers to vaccination and implementing or scaling up known best practices.
It is expected that these combined initiatives will contribute to improving vaccine access and uptake in Canada to reach our national vaccine coverage goals and disease reduction targets by 2025.
| Province/Territory | Web information |
|---|---|
| British Columbia | British Columbia ImmunizeBC |
| Alberta | Alberta Health Services Immunization |
| Saskatchewan | |
| Manitoba | |
| Ontario | |
| Quebec | Vaccination |
| New Brunswick |
|
| Nova Scotia | |
| Prince Edward Island | |
| Newfoundland | Newfoundland Labrador Health and Community Services Immunization |
| Yukon | Yukon Health and Social Services Immunization Information |
| Northwest Territories | NWT Health and Social Services Immunization/Vaccination |
| Nunavut | Nunavut Department of Health Immunization |
D1: National laboratory system
Joint external evaluation target: Real-time bio surveillance with a national laboratory system and effective modern point-of-care and laboratory-based diagnostics.
Level of capability in Canada
This report focuses on microbiology laboratories responsible for testing of biological samples. The laboratory system in Canada includes facilities at the national level, within each of the 13 provinces and territories, and at the local level. The National Microbiology Laboratory (NML) currently has four lab-based facilities.
At the provincial level, most jurisdictions have an officially designated provincial public health laboratory that often operates with close linkages to post-secondary institutions to support education and the advancement of research. The territories work closely with nearby provincial laboratories for the testing of their samples. Some provinces have laboratories that are not officially designated as provincial public health laboratories.
Within each jurisdiction, the local level laboratory system is comprised of hospital acute care microbiology laboratories and community laboratories (e.g. private laboratory providers). Smaller jurisdictions that do not possess the laboratory infrastructure can access other provinces as reference laboratories, in addition to accessing the NML.
A Canadian Public Health Laboratory Network Strategic Plan 2016-2020Footnote 4 for federal and provincial public health laboratories has been in place since 2002. It is updated every three to five years by the Canadian Public Health Laboratory Network, a standing national expert group of the Pan-Canadian Public Health Network.
Canada possesses a high level of competency to maintain access to and conduct laboratory testing for a large number of communicable diseases. The NML has identified approximately 300 tests that can be conducted, which includes the four from the International Health Regulations immediately notifiable list. One hundred percent of the population has access to laboratory services for testing priority diseases; however ease of access can vary depending on the geographic location of the affected community.
Clinicians across the country routinely use the laboratory system. They can access services through community laboratories or through hospital acute care microbiology laboratories that will refer to provincial laboratories when required. Protocols and guidelines for communicable disease investigation exist at all levels of government for a variety of diseases (e.g. requirements for sampling and submission, for specific recommended tests and shipping precautions, or for submitting isolates or samples for confirmation and/or further characterization).
Most tertiary centre or public health laboratories have a web-accessed guide to services describing a menu of available tests and sample requirements, which is available to clinicians. Laboratory reports are routinely provided by a number of different methods to ordering clinicians. Similarly, at the national level, results are provided back to the referring provincial health laboratory/hospital via the client's preferred method (i.e. hard-copy mailed, emailed, or faxed). Canada is working to develop a system of electronic test requisitioning and reporting. Each test performed at the NML has a test-specific turn-around time stated in the NML's Guide to Services.
At the provincial and territorial level, some laboratories use specified turnaround times for their tests as part of their quality system accreditation. These times will vary depending on the complexity of the test and urgency of the request. Wherever possible, electronic methods are used to facilitate timely reports back to clinicians.
It is important to recognize that Canada's provincial and federal public health laboratories do not operate in isolation. They work together as equal partners within the Canadian Public Health Laboratory Network to:
- Address nation-wide laboratory diagnostic standards, emergency preparedness and response
- Develop and implement a long-term vision for public health laboratories
- Strengthen and assure the capacity and capabilities of Canada's public health laboratory system
For example, the Network is responsible for drafting and updating the Laboratory Annex to the Canadian Pandemic Influenza Plan.
Indicators
D.1.1 Laboratory testing for detection of priority diseases
Testing capabilities
The provincial and federal public health laboratories all have a reference laboratory function. Core tests for priority diseases are implemented effectively across the tiered laboratory network. Laboratories in Canada operate through a four-tier system where health centre laboratories make up the first tier and support front line primary health care. In the second and third tiers are hospital and regional reference laboratories (i.e. provincial public health laboratories), respectively, and the fourth tier is a national reference centre (i.e. the National Microbiology Laboratory).
Although in Canada neither the NML nor the provincial laboratories have identified 10 specific diseases to test, each has sufficient capacity to conduct a large number of tests, including those that are most frequently required in the country or within individual provinces or territories. The laboratory resources of provinces and territories and the NML provide sufficient capacity to test for a broad range of samples, including the World Health Organization's (WHO) top 10 priority diseases and most known microorganisms. Organizations such as the Centers for Disease Control and Prevention (CDC) in the United States provide capacity for testing exceedingly rare diseases.
Across the provinces and territories, there are enough reference laboratories to collectively support the testing of all classes of microbial pathogens. For instance, in New Brunswick, there are three facilities: CHU Dumont for viruses; Saint John Regional Hospital for enterics and tuberculosis; and Dr. Everett Chalmers Hospital and CHU Dumont for mycology service.
Jurisdictions that do not have such facilities use reference laboratories in neighbouring provinces.
The NML and, in some cases, the CDC also provide reference laboratory services upon request.
Use of personal protective equipment
At the provincial and territorial level, the availability of personal protective equipment (PPE) for Canadian laboratories in general is tracked through basic inventory control locally/regionally. Laboratories are responsible for tracking and ensuring they have an adequate supply of PPE. In many jurisdictions, this is required for accreditation.
The training procedures for PPE use in laboratories in Canada vary according to jurisdiction, but all federally licensed facilities include a biosafety training program. PPE is available for laboratory staff across the country in accordance with workplace health and safety requirements. At the national level, the NML tracks inventory of positive pressure suits through a suit management program. These suits are used for working with RG4 pathogens.
The NML also manages a respirator fitting and management program for CL3 users and training on the donning and doffing of PPE.
Standardization of diagnostic testing
Canada does not have standardized diagnostic testing since federal, provincial and hospital laboratories perform different types and volumes of testing. The NML participates in external quality assessment programs and also distributes proficiency panels to other laboratories both routinely and during emergent or potentially emergent events (such as SARS, MERS, H1N1, H7N9, H5N1, Ebola Zaire, and Zika).
High- and medium-consequence communicable disease testing is fairly well standardized across the country. All provincial public health laboratories and the NML are members of the Canadian Public Health Laboratory Network, where coordination and standardization of testing is managed and done where applicable. This applies, for example, to approaches for detecting agents such as pandemic or seasonal influenza, Zika or Ebola during an outbreak or public health threat.
For most other medical laboratory testing, the technologies, indications and standard investigative pathways used varies across laboratories but are largely based on Health Canada approved diagnostic platforms or international and national association guidelines.
At the provincial/territorial level, laboratories ensure standardization of testing within their jurisdiction through a variety of methods. These include reference testing against external facilities, or coordinating with provincial partners to ensure standardization. For example, in Newfoundland and Labrador, partnerships with provincial stakeholders allows for best practices to be shared and clinical oversight to be standardized. Additionally, all laboratories in Newfoundland and Labrador are accredited by the Institute for Quality Management in Healthcare and participate in proficiency testing through the Institute, the College of American Pathologists, Clinical Microbiology Proficiency Testing, and the NML.
Biosafety and biosecurity training
Biosecurity awareness training is provided to all laboratory staff. Biosafety training is based on a training needs assessment and can include:
- General lab safety
- Biosafety cabinet use
- Autoclave training
- Biological spill clean-up training
- Transportation of dangerous goods
- CL-3 laboratory training, emergency and incident response training
- CL-4 laboratory training
Training needs vary by jurisdiction and required training is provided by individual organizations.
Under section 9 of the Human Pathogens and Toxins Regulations, biosafety officers are responsible for arranging appropriate training related to applicable biosafety and biosecurity standards. Section 4.3 of the Canadian Biosafety Standard outlines training program standards. The Public Health Agency of Canada, as a regulator, provides training resources to aid stakeholders in completing their biosecurity and biosafety training program requirements.
Through the Agency's e-learning portal, stakeholders have access to 22 courses and three instructional videos. All microbiology laboratories in Canada are required to have biosecurity and biosafety plans, and annual review of biosafety protocols are required as part of the laboratory quality system accreditation.
D.1.2 Specimen referral and transport system
Transportation of clinical and public health specimens
Canada does not have formally documented specimen referral routes for detection/confirmation of top 10 priority diseases. Most specimen transport requires a combination of road and air travel. Canada does follow required Transportation of Dangerous Goods Regulations and the International Civil Aviation Organization standards when transporting samples into or out of Canada and within Canada.
Federal oversight of public health specimen referral
While there is no formal specimen referral network at the federal level, instructions for referring samples are clearly posted and updated as required within the NML's Guide to Services. In addition, the Human Pathogens and Toxins Regulations cover the biosecurity aspect of sample referral, while the Transportation of Dangerous Good Regulations covers the transport safety aspects of sample referral.
Canada participates in many national and international laboratory networks, including:
- Canadian Public Health Laboratory Network
- Canadian Food Safety Information Network
- Global Health Security Initiative
- Global Heath Security Agenda
- Biosafety Level 4 Zoonotic Network
- Other agent-specific or thematic networks and organizations around the world
The NML also supports or has supported laboratory network development initiatives in:
- Laos, Cambodia, Vietnam and the Philippines, as part of the Canada-Asia Regional Emerging Infectious Disease Project
- Ukraine and the Caribbean region through the Caribbean Public Health Laboratory Network and the Caribbean Vector Borne Disease Network
Plan for transporting specimens safely throughout the country
The Transportation of Dangerous Goods Regulations provide for the transport of specimens classified as infectious substances. They outline requirements for dangerous goods training and classification, packaging, labelling and documentation of infectious substances, as well as for emergency response assistance plans.
Canada has no formal plan for transporting specimens given that it is a federated nation where authority over public health resides with the provinces and territories. There is, however, a global understanding of how the country's tiered public health system and its laboratories operate and how routine shipping of samples operates.
Each jurisdiction predetermines transport pathways and determines final destination labs. During outbreaks, specific issues concerning movement of samples and diagnostic protocols and processes are discussed, approved and executed in very short order.
The NML is the national coordinator for emergency response assistance plans, which describe what is to be done in the event of a transportation accident involving certain higher risk dangerous goods. In the case of laboratories, this means RG4 pathogens. A Plan is required under the Transportation of Dangerous Goods Regulations for dangerous goods that require special expertise and equipment to respond to an incident.
D.1.3 Effective modern point of care and laboratory based diagnostics
Point-of-care testing is provided through a number of methods and in many locations, which vary by jurisdiction.
- Health Canada sets performance standards for products that can be marketed in Canada. These provide provinces and territories with a minimum-performing toolbox of point-of-care testing and laboratory-based diagnostics to use.
- Reference laboratories design and validate in-house methods in most jurisdictions to meet laboratory, diagnostic and public health needs that are not covered by Health Canada approved products.
- Most jurisdictions have several large laboratory centres fed by regularly scheduled transportation feeds. They are capable of handling the majority of specimen types and in large volume.
- Modern instrumentation has close to 100% function with support from modern information technology networks.
Many jurisdictions have standards for point-of-care testing and have developed procedures and training for this type of testing. In some provinces and territories, there are plans to build capacity for point-of-care diagnostics at clinical sites. In Quebec, for instance, each institution has developed its own medical biology services organization in an attempt to offer as much analysis as possible within its own facilities. The OPTILAB project was developed to improve and streamline these services in terms of timeliness, information systems and other key elements.
Procurement of necessary media and reagents to perform core laboratory tests is mainly done locally or provincially. For example, British Columbia's public health laboratory makes some of its own specialized media on site, and procures the rest via a provincial procurement system. In Alberta, procurement is done by individual labs for private diagnostic laboratory services and the Regional Health Authority is responsible for procurement of media and reagents for the provincial laboratory services.
Federal level laboratories are subject to guidelines and are responsible for selection of the equipment, reagents, and consumables that they require. The federal government has extended access to the Federal Buy and Sell procurement initiative to provinces, making country-wide procurement an achievable goal.
Although the NML has taken the lead on evolving technologies such as Whole Genome Sequencing and bioinformatics, these examples demonstrate how laboratories in Canada meet standards or lead the technology wave by working together as a network.
D.1.4 Laboratory quality system
Licensing and inspection
Licensing and inspection of most laboratories is also a function of provincial governments or provincial government agencies, and is linked to accreditation. While licensing of laboratories is mandatory, each province sets out its own individual licensing and inspection expectations as there is no national oversight. The exception is for microbiology laboratories, which must be licensed as well by the Public Health Agency of Canada. The Agency also conducts regular inspections that ensure that proper safety practices are observed and that security measures align with planned functions.
Federal laboratory facilities are typically excluded from provincial licensing and accreditation oversight, but not Federal Office of Biosecurity oversight. Federal facilities voluntarily subject themselves to Standards Council of Canada/International Organization for Standardization (ISO) accreditation. However, safety inspections are mandatory.
The Standards Council of Canada accredits the NML's ISO-based quality systems and assesses them at a minimum, biennially. The Public Health Agency of Canada licenses NML's use of pathogens and, as the regulator, is the national body in charge of laboratory licensing and inspection for biosafety and biosecurity under the Human Pathogens and Toxins Act and the Health of Animals Act and their associated regulations. In conjunction with the Canadian Food Inspection Agency's Office of Biohazard Containment and Safety, they are responsible for assessing NML's biosafety/biosecurity.
Inspections of the NML are done via an on-site visit in which all clauses of the standard are assessed by a team including quality management system and technical experts. In general, the Public Health Agency of Canada, as the regulator, uses a risk-based approach in selecting the Human Pathogens and Toxins Act licenced laboratories to inspect and the frequency of inspection, which takes into consideration key risk factors including:
- Dangerous pathogens at a minimum
- RG4 inspected annually
- RG3 inspected once within every three years
- Compliance history
- Robustness of laboratory's biosafety program and management
- Complexity of work conducted
- New and emerging biosafety and biosecurity risks
- External risk identification (e.g. information could originate from other regulatory departments, intelligence or police services, or anonymous sources)
- Document review (including the Plan for Administrative Oversight, biosecurity plan, standard operating procedures)
Certification and accreditation
The Standards Council of Canada is the national body responsible for laboratory certification (using ISO 9001), and laboratory accreditation (using ISO 15189). The NML is accredited for disease-specific testing by the World Health Organization. Its diagnostic tests have accreditation based on ISO 15189, ISO 17025 and ISO 17043 as well as certification to ISO 9001.
The NML has a rigorous quality management system under which policies, processes and procedures are documented and followed. This laboratory-based quality management system is regulated by the Standards Council of Canada, which also regulates the initial accreditation phase for the proficiency panels that NML distributes. All accredited tests are required to undergo annual external proficiency tests or, if these are not available, an inter-laboratory comparison. Health Canada has two reference laboratories for botulism and Listeria that are both certified to ISO 17025 standards.
In provinces and territories, laboratories are accredited through a number of organizations including:
- Provincial and territorial colleges of physicians and surgeons
- College of American Pathologists
- Accreditation Canada
- Canadian Association for Laboratory Accreditation Inc.
- Institute for Quality Management in Healthcare
For example, in Alberta all public and private clinical diagnostics labs must be accredited by the College of Physicians and Surgeons of Alberta, whose quality standards are similar to those of the College of American Pathologists and IS 15189 lab standards. Proficiency testing is an important and thoroughly evaluated requirement of these standards. Some laboratories are also College of American Pathologists accredited to quality for accreditation by an internationally recognized accreditation body.
For most community-level laboratories there is no national body responsible for laboratory accreditation and no national external quality assessment program in bacteriology, virology, serology, or parasites. In Nova Scotia and Prince Edward Island, microbiology laboratories use Clinical Microbiology Proficiency Testing for bacteriology and parasites and the College of American Pathologists for serology and virology.
Medical device (in vitro reagent) registration and regulation
The Health Canada, Medical Devices Compliance Unit is the national body responsible for regulating the process of in vitro diagnostics device (e.g. reagents) qualification or registration. Manufacturers must apply for a license for testing kits as per the documented processes before legally marketing their products in Canada. Programs do exist that allow laboratories access to as-yet unqualified or unregistered products that have not yet sought approval in Canada.
Supervision plan and procedures
In addition to NML inspection, certification and accreditation, supervision is provided through the WHO and the Pan American Health Organization. In jurisdictions across provinces and territories, other supervision is provided. For example, in Manitoba there is legislation to report specific organisms to the province and to report RG3 organisms to the federal government.
At the national level there are standardized checklists for internal audits, external assessments and inspections. Laboratories receive a report after each supervisory event, and there are indicators to measure the progress in laboratory test quality. Similarly, within some jurisdictions there are plans and procedures in place for quality assurance oversight.
Quality assurance is typically ensured through accreditation and is overseen by the local medical college. For instance, in Alberta external quality assessment and proficiency testing is a component of the overall quality system of the provincial College of Physicians and Surgeons and the College of American Pathologists. A lab that does not score 100% undergoes a mandatory corrective action, which is required under the very stringent quality system. This is a requirement for maintaining College of American Pathologists and provincial College of Physicians and Surgeons accreditation.
A number of jurisdictions also have standardized supervision checklists or procedures in place. Newfoundland and Labrador have internally developed procedures that are based on the Institute for Quality Management in Healthcare standards. These are completed periodically by region by trained internal auditors. These are monitored through site inspections and through certification and proficiency testing. Supervision is provided through the Institute for Quality Management in Healthcare, the Public Health Agency of Canada, and other oversight bodies.
National external quality assessment programs
Canada has a national external quality assessment program in the areas of bacteriology, virology, serology and parasitology. In addition, Health Canada provides national reference diagnostic services for Listeria, botulism and Vibrio, and houses additional networks for virology and parasitology. These include:
- The Listeria Reference Laboratory: provides investigation of listeriosis cases, maintenance of a national collection of isolates. and houses a comprehensive molecular epidemiological database of all isolates in Canada for use as a resource for outbreak investigations, research and other microbiological investigations
- The Botulism Reference Laboratory: helps investigate suspected outbreaks of botulism across the country, and analyses bacteria spores and toxins found in clinical and food samples from across the country
- The Food Virology Reference Laboratory: a resource available to Canadian laboratories testing clinical, environmental or food samples for foodborne viruses
- The Vibrio Reference Laboratory: provides comprehensive reference and research laboratory services, and is developing next generation laboratory methodologies to enhance the national surveillance, clinical diagnosis and outbreak response capabilities
- The Food and Environmental Virology Network
- The Food and Environmental Parasitology Network
- ViroNetwork
All reference services handle clinical samples when required.
While there is no national requirement for federal laboratories to participate in an external quality assessment program, the NML participates in such programs because it is good practice. If assessment results are not satisfactory, or if there are outliers, they are investigated for root cause and a corrective action plan is developed and implemented until the laboratory is satisfied that the test method is performing as expected.
Participation in external quality assessment programs is mandatory for public and private laboratories at the provincial and territorial level. Although at the local or community level, participation in an external quality assessment program is not required, many of these laboratories still choose to participate. In most jurisdictions, follow-up corrective action with documentation is implemented when assessment results are not satisfactory.
Supervision and quality assurance for surveillance programs
Canada uses many surveillance programs to inform its public health interventions. The generation of good quality laboratory data is essential not only for clinical intervention, but it is also the foundation supporting the correct epidemiological investigation and public health surveillance. The following is a sampling of some of the surveillance programs in which laboratories engage:
- PulseNet Canada
- Canadian Integrated Program for Antimicrobial Resistance Surveillance
- Canadian Measles/Rubella Surveillance System
- Canadian Pediatric Surveillance Program
- Creutzfeld-Jacob Disease Surveillance System
- Enhanced National Enhanced Pneumococcal Invasive Disease Surveillance System
- FluWatch
- FoodNet Canada
- HIV/AIDS Surveillance
- International Circumpolar Surveillance System
- Invasive meningococcal disease: Monitoring
- National Enteric Surveillance Program
- Respiratory Virus Detection Surveillance System
- West Nile Virus Surveillance
For more information on surveillance programs in Canada, see section D2: Real-time surveillance.
Best practices, challenges, gaps and recommendations
Part of the robust nature of the national laboratory system in Canada is attributed to the Canadian Public Health Laboratory Network. It has responded as a network to outbreaks such as SARS, H1N1, Ebola, and Zika and has also been prepared to respond to pathogens that did not severely impact Canada (MERS-CoV, H7N9, H5N1, etc.). The Network laboratories have consistently worked together to improve diagnostic standards relating to emerging threats affecting their laboratories (AMR, Lyme, Syphilis, etc.), and have worked to improve agreements on collaboration (sample sharing, PulseNet Canada/Canadian Laboratory Surveillance Network) to streamline system performance. Moreover, the Network is now working to establish and implement a strong vision for advancing diagnostic technologies in Canada, which will include whole genome sequencing and bioinformatics, as well as transitioning to culture independent diagnostic testing where appropriate.
As the landscape of public health laboratory science continues to evolve, a notable challenge is the transition to new technologies such as whole genome sequencing. Canada's challenge is that there are too many options rather than too few. It is difficult to standardize and align while all laboratories face two primary categories of challenges: managing an increase in the types of pathogens Canada sees every year, while needing to evolve diagnostic technologies at the same time.
Work is underway to ensure accurate, reliable and timely laboratory diagnostic techniques to properly identify microbial causes of illness and inform on patient care. Another priority for the Canadian Public Health Laboratory Network is to develop approaches to address gaps in training, capacity and data analysis related to whole genome sequencing and other advanced technologies.
D2: Real-time surveillance
Joint external evaluation target: Strengthened foundational indicator- and event-based surveillance systems that are able to detect events of significance for public health, animal health and health security; improved communication and collaboration across sectors and between sub-national, national and international levels of authority regarding surveillance of events of public health significance; improved country and regional capacity to analyze and link data from and between strengthened, real-time surveillance systems, including interoperable, interconnected electronic reporting systems. This can include epidemiologic, clinical, laboratory, environmental testing, product safety and quality, and bioinformatics data; and advancement in fulfilling the core capacity requirements for surveillance in accordance with the International Health Regulations (IHR) and the World Organization for Animal Health standards.
Level of capability in Canada
Canada has strong public health surveillance systems in place to detect and monitor existing and emerging disease and events of significance to human health, animal health and health security. These systems are able to act upon, communicate and share information across authorities, jurisdictions and sectors.
Public health surveillance is a shared responsibility among federal, provincial, territorial and local levels of government in collaboration with the private sector, non-governmental organizations (NGOs), health institutions, and health professionals and their associations. The Public Health Agency of Canada (PHAC) is the focal point for public health surveillance at the federal level.
Local, provincial and territorial health authorities are the major contributors of data for national level surveillance. Canada leverages a complex system of epidemiological, laboratory, policy and program, and corporate support activities to carry out its public health surveillance function. Canada relies on collaborative, interoperable and electronic systems to ensure that the national surveillance meets the public health needs of Canadians and fulfills national and international requirements and agreements.
Indicators
D.2.1 Indicator-based and event-based surveillance systems
Examples of national event-based surveillance systems
- The Pan-Canadian Public Health Network (PHN) is a key intergovernmental mechanism that supports Canada's ability to anticipate, prepare for, and respond to public health events and threats. The PHN supports a collaborative federal/provincial/territorial approach to address public health emergencies and assists jurisdictions in gaining a stronghold on public health issues. The Public Health Network Council establishes Special Advisory Committees to respond to specific significant public health events.
-
The Global Public Health Information Network (GPHIN) supports Canada's Health Portfolio, provincial and territorial governments, and the global health security community, including the WHO and the Pan American Health Organization, through the provision of timely and comprehensive public health intelligence and early threat detection. The network tracks events such as disease outbreaks, contaminated food and water, bio-terrorism and exposure to chemicals, natural disasters, and issues related to the safety of products, drugs and medical devices. It provides early detection and alerting, analysis, risk assessment, and communication of potential and emerging threats to human health.
Global Public Health Information Network collects data daily (24/7), analyzing news wires, media reports, government posts and other open-source content from more than 20,000 online sources in nine languages worldwide. It fulfils Canada's IHR core capacity obligation for event-based surveillance and supports global health and health security communities through its capacity for early threat detection.
-
Canadian Network for Public Health Intelligence (CNPHI) Public Health Alerts was developed and is managed by the National Microbiology Laboratory with guidance from multi-jurisdictional working groups. The Network is a secure, scientific, public health informatics and bio surveillance platform. It leverages the integration of disparate public health information resources and expertise for the direct benefit of local, regional and national decision makers.
The platform fosters collaboration and consultation through mechanisms for disease surveillance, intelligence exchange, and research and response to protect, promote and support public health. Innovative solutions such as advanced aberration detection systems, rapid and adaptive data collection, interactive data interrogation, and data visualization have been integrated into numerous national surveillance programs.
-
PulseNet Canada (PNC), led by the National Microbiology Laboratory, is the only national real-time molecular subtyping network for foodborne disease surveillance supporting the rapid detection and response to single- and multi-jurisdictional outbreaks. PNC has linkages to the National Enteric Surveillance Program and FoodNet Canada sentinel surveillance. (See section P5: Food safety for more information.)
PNC specifically focuses on outbreak detection and response by collecting and using daily molecular/genomic data from cases of bacterial foodborne disease from all provincial public health laboratories, and from bacterial pathogens isolated from the Canadian Food Inspection Agency, in real-time.
Data are analysed and assessed weekly in conjunction with the National Enteric Surveillance Program to determine which increases in illnesses represent related clusters that should be investigated as outbreaks. PNC also provides laboratory investigation during multi-jurisdictional outbreak response and support for single jurisdiction response to enable timely public health action.
FoodNet Canada data (molecular and genomic) are also analyzed by PNC to ensure data from both systems provides value and context to the other. Work of PulseNet Canada has been critical for the detection and response to large national outbreaks including listeriosis in 2008, E. coli O157 in 2012, and Salmonella related to chia products in 2014.
- Laboratory Incident Notification Canada and Laboratory Exposure Surveillance System support reporting and notification requirements under the Human Pathogens and Toxins Act and the Human Pathogens and Toxins Regulations. PHAC must be notified of:
- An inadvertent release or production of a human pathogen or toxin
- Any incident involving a human pathogen or toxin that has caused, or may have caused disease, in an individual
- A pathogen or toxin that has been stolen or is otherwise missing
PHAC receives notifications through an online reporting module that is available for secure creation, storage and submission of laboratory incident reports through the biosecurity portal. The Laboratory Incident Notification Canada online portal is monitored by PHAC, as a regulator, for trends and root cause analysis. If further information is required, PHAC contacts the regulated party by phone, email or fax, as deemed most appropriate.
- Canadian Adverse Events Following Immunization Surveillance System is a federal/provincial/territorial public health post-market vaccine safety monitoring system. The Surveillance System continually monitors safety of vaccines within Canada. Its purpose is to identify increases in the frequency or severity of previously identified vaccine-related reactions, unknown adverse events following immunization, and areas that require further investigation and/or research. It also provides timely information on the reporting profiles of adverse events following immunization for vaccines marketed in Canada that can help inform immunization related decisions.
- Health Canada's Radiation Protection Bureau operates the Fixed Point Surveillance Network, a cross-Canada real-time environmental radiation monitoring network with public reporting that is capable of detecting increases in radiation from natural or anthropogenic sources that could lead to a potential radiation emergency (see section R2: Emergency response operations).
- The Health Portfolio Operations Centre serves as Canada's public health single window and the platform for planning and managing the coordination of response activities to significant public health events of national interest. (See section R2: Emergency response operations.) The Health Portfolio Operations Centre supports epidemic intelligence that provides early warning and rapid response to national and international public health threats.
- Public health surveillance activities during mass gatherings require multi-jurisdictional collaboration to support ongoing situational awareness, quick detection of sporadic infectious disease activity, outbreak activity, environmental health hazards and major health events. For example, when Canada hosted the Toronto 2015 Pan/ParaPan American Games, 15 surveillance partner organizations collaborated (e.g. organizing committee, PHAC, provincial government agencies, and local public health informatics teams, public health units and laboratories).
- Canada has provided international support to surveillance system development and implementation. For example, in 2014, PHAC mobilized epidemiological support to Trinidad to develop surveillance protocols for communicable disease for cruise lines and hotels. Also, in response to the Ebola outbreak in Africa in 2015, epidemiologists were deployed to Senegal to support capacity-building activities related to surveillance and outbreak investigations. Further, Canada has participated actively in the development of the Epidemic Intelligence from Open Source (EIOS) platform, the WHO Health Emergencies Programme's event-based surveillance initiative.
Examples of national indicator-based surveillance
- In Canada, disease surveillance begins at the local, provincial and territorial public health levels. The national notifiable disease list and associated case definitions are developed and revised based on federal/provincial/territorial consensus using established criteria. Each province and territory identifies a list of diseases or health conditions that are required to be reported within that jurisdiction. The reportable disease lists may also mandate reporting at different rates, where some may be considered as real-time or near real-time reporting. The purpose of making a specific disease reportable is to facilitate tracking and control efforts by public health within the jurisdiction.
- In addition, provinces and territories voluntarily submit data on approximately 60 nationally notifiable diseases to the Canadian Notifiable Disease Surveillance System on an annual basis (see Annex A). The System is operated at the national level to collect, validate, and maintain surveillance data on conditions deemed nationally notifiable. Although not a real-time surveillance system, it provides historical and baseline trend data on nationally notifiable diseases and data is used to report annually on national counts and rates of these diseases.
-
Canada also conducts national enhanced surveillance on many of the nationally notifiable diseases, such as measles, invasive meningococcal disease and influenza. Many other surveillance systems run in parallel and near-real time, for example the National West Nile Virus and Other Mosquito Borne Diseases surveillance system.
Others are slower moving systems that provide important information on priority diseases, such as the Canadian Tuberculosis Reporting System and the HIV/AIDS surveillance system. These activities vary in scope and in the degree to which they encompass the full cycle of surveillance. Some are long-standing systems that operate on a continuous basis, while others are new or serve cyclical or seasonal purposes.
- National real-time surveillance is only required for Canada's high priority diseases. Otherwise, real time surveillance is a provincial and territorial responsibility to address jurisdictional need. However, the federal government is able to provide support for multi-jurisdictional or pan-Canadian outbreaks, when requested/required.
- For Canadian Armed Forces (CAF) populations (in Canada and deployed internationally), the Canadian Forces Health Services requires specific communicable disease cases or symptoms to be reported centrally. Diseases reported include those that are on Canada's nationally notifiable disease list as well as diseases relevant to the CAF circumstance. Within Canada, a notifiable disease that occurs to a CAF member also requires CAF reporting to the local public health authority and is subject to provincial and territorial regulation. Outside Canada, during deployment, there is regular monitoring of health encounters through the Disease and Injury Surveillance System.
Data validation and quality assurance
In addition to data validation and quality assurance systems with local, provincial and territorial stakeholders, PHAC continues efforts to improve data quality. Each surveillance system develops its own internal data validation processes appropriate to the data and exchange mechanism used. Internal validation generally serves to ensure that:
- Data is within the acceptable date range for the set
- Age ranges for cases are appropriate
- There are no missing values for key elements
- The dataset includes only cases of diseases/events targeted by the system
External validation can include steps such as referral back to provincial and territorial data providers and allow for updates or corrections where necessary.
PHAC has also developed a number of overarching policies, guidelines and initiatives to support internal data quality and data management. PHAC continues to move towards a more consistent model for data management through enhanced governance for data acquisition and operations, data quality, data security and technical infrastructure.
D.2.2 Interoperable, interconnected, electronic real-time reporting system
Examples of electronic surveillance systems and interoperability
Electronic health information system capacity varies across jurisdictions. Each jurisdiction has a different combination of processes and mechanisms for the receipt of indicator or event-based data from its jurisdictional data sources (physicians, local public health authorities, and laboratories) and includes paper reporting, telephone, facsimile and electronic reporting. Infoway reports an average of 73% adoption of Electronic Medical Records among primary care physicians in 2015, though the rate varies by province or territory.
In Alberta an electronic reporting application is used to provide the provincial government with access to real time data that informs policy development and decision making. Similarly, in Saskatchewan, local public health units report human communicable disease cases via an electronic application (integrated Public Health Information System.
Examples of national electronic systems include:
-
CNPHI, a scientific public health informatics and biosurveillance platform that can support collaboration, surveillance, alerting, knowledge management, laboratory support, and event management among a large number of public health professionals across the human, animal, and environmental health sectors. Users include epidemiologists, lab scientists, physicians, nurses, policy groups, and others. CNPHI is recognized as providing key foundational infrastructure for public health surveillance in Canada.
Launched by PHAC in 2004 with an Alerts system, the platform currently has six focus areas covering: Knowledge Management; Surveillance; Alerts; Collaboration; Event Management; and Laboratory Systems.
- PulseNet Canada, which provides a virtual electronic network tying together the public health laboratories of all provinces (plus the federal laboratories) by linking their computers and databases, collects data from cases of bacterial foodborne disease from all provincial public health laboratories, and from bacterial pathogens isolated by the Canadian Food Inspection Agency to quickly identify and respond to foodborne disease outbreaks.
- The Canadian Notifiable Diseases Surveillance System receives annual provincial and territorial data (either case-level or aggregate), through a secure exchange of electronic datasets. However, the format of these datasets is determined by the reporting jurisdiction. Once received, all of the submissions are standardized into a common format to allow for analysis and reporting.
The extent to which real-time electronic public health surveillance systems are interoperable and interconnected varies by jurisdiction. At the provincial and territorial level, while most electronic reporting systems are independent, there is some opportunity for sharing between sectors through established protocols such as the CNPHI scientific informatics platform.
The interoperability of surveillance systems is also controlled by privacy legislation, which varies by jurisdiction. In part, privacy protection and confidentiality are achieved by ensuring that surveillance systems are only connected when appropriate/necessary. Although not all surveillance systems are continuous real-time systems, one can be put into place if needed.
In general, PHAC's surveillance systems are not shared between sectors and operate primarily within the public health domain. At the national level, aggregate surveillance data may be shared with other government departments and stakeholders to fulfil program and policy needs.
- National infectious disease surveillance systems developed using the CNPHI platform are able to support the sharing of integrated, non-nominal laboratory and epidemiological data in close to real time.
- At the National Microbiology Laboratory, an electronic requisitions and reporting system is currently in development to support electronic reporting of laboratory test results to the client laboratories to support timely clinical decision-making and case confirmation efforts.
- The Canadian Notifiable Diseases Surveillance System is a static electronic database that receives annual data submissions from reporting jurisdictions.
- Canada Health Infoway has partnered with federal, provincial and territorial governments and other organizations on more than 400 projects. These include the development of solutions for public health surveillance. For example, Panorama is a system designed to support public health case management, the identification of infectious disease outbreaks and the management and control of vaccine inventories and immunization programs. The system provides public health professionals with integrated tools that assist in monitoring, managing, and reporting on public health. It also provides front-line service providers and public health decision makers with critical information and reuses centralized data where possible.
In Canada, animal surveillance for the detection of disease outbreaks and control programs is a well-structured and effective risk-based program. Remote area surveillance and data handling mechanisms could be improved with the use of integrated databases to provide more timely and enhanced information on real time changes to the animal health situation.
- Canadian Food Inspection Agency establishes policies and standards for the biosecurity of animal health in Canada with policies and requirements for the import of livestock, animal products and food safety. The Canada Border Services Agency enforces these policies and standards procedures.
- Canadian Notifiable Avian Influenza Surveillance System is a joint initiative of government, industry and Canadian farmers to prevent, detect, and eliminate the presence of notifiable avian influenza in Canada's domestic poultry flocks. Surveillance data and information from all avian influenza surveillance activities are validated to test the effectiveness of the passive surveillance and detect circulating low pathogenic avian influenza in domestic poultry.
- The Canadian Food Inspection Agency has established consultation mechanisms with industry associations such as the Canadian Animal Health Surveillance System, which has no direct control from government or any one group. It does, however, include individual network groups that are self-organizing, self-funding and self-governing. Their shared purpose is to strengthen animal surveillance.
- PHAC collaborates with the Canadian Food Inspection Agency on the surveillance of rabies and avian influenza. PHAC also collects data on the incidence of food-borne illness, including those of animal origin, through the National Enteric Surveillance Program (see section P4: Zoonotic disease).
Reporting to national and regional stakeholders and interconnectedness
As mentioned above, the PHN is a network of representatives across Canada covering many sectors and levels of government, who work together to strengthen public health in Canada. The work of the PHN is governed by the Pan-Canadian Public Health Network Council composed of federal, provincial and territorial government officials from all jurisdictions, who are responsible for public health. The PHN Council is accountable to the Conference of Federal, Provincial and Territorial Deputy Ministers of Health which provides direction and approves public health priorities for Canada.
- The Blueprint for a Federated System for Public Health Surveillance in Canada is a framework designed to help guide federal, provincial and territorial governments in moving towards an interconnected model for public health surveillance, recognizing the need for national coordination.
- The Multi-lateral Information Sharing Agreement is a legal agreement (see P1: National legislation, policy and financing for further details) that provides the authority for the exchange of information for:
- Surveillance of infectious diseases (which includes routine surveillance, and case management and response activities)
- Management of pan-Canadian and multi-jurisdictional public health events
- Public health emergencies of international concern.
PHAC depends on the voluntary cooperation of provincial and territorial authorities for health surveillance (including case reporting) and response to outbreaks. Existing legislation does not specify terms for inter-jurisdictional cooperation. However, policy statements, intergovernmental agreements and memoranda of understanding have traditionally been used to formalize the terms of intergovernmental collaboration.
The Canadian Notifiable Disease Surveillance System sends annual reports of aggregate data and basic trends, comparing the reporting jurisdiction to the national level. The System does not report directly to intermediate or regional stakeholders, Aggregate data are shared with national disease program areas within PHAC, routinely or upon request, on notifiable diseases of interest to them.
The National Microbiology Laboratory has in place standard operating procedures for the reporting of high consequence laboratory results to national and provincial public health stakeholders to support infectious disease surveillance and outbreak response efforts (e.g. Ebola virus, Zika virus, emerging respiratory pathogens, and other potentially high-consequence test results). Responsibility for regional reporting rests largely at the provincial level.
Public reporting of surveillance data
In the provinces and territories, the main approach for jurisdictions to report to the public is through their respective provincial or territorial government websites and media releases.
- In Quebec, the preferred method to communicate with the public is via the provincial health department web portal, where the public and health professionals can find information on notifiable diseases and public health issues.
At the national level, while each surveillance program or activity has its own unique reporting practices, PHAC's surveillance outputs are generally circulated directly to data providers (provinces and territories) and relevant stakeholders and published to the general public on Diseases and Conditions. For example:
- Weekly surveillance reports and monitoring maps are published on Canada.ca for Measles and Rubella and FluWatch. For FluWatch. Aggregate data is shared with provincial and territorial public health departments and sentinel sites to assess the intensity of influenza circulation. National data is shared with the WHO to contribute to mapping the global circulation of influenza.
- Travel advice and advisories are published online and provide the public with official information and advice from the Government of Canada on situations that may affect safety and well-being. Also, the Committee to Advise on Tropical Medicine and Travel is an expert advisory body that assists PHAC with travel health-related advice for travellers and health care professionals.
- The Canadian Communicable Disease Report is a scientific, peer-reviewed journal published online in French and English. The Report, published by the Public Health Agency of Canada, is geared to both clinicians and public health professionals and includes the latest statistics on infectious disease trends in Canada, advisory committee statements, original research, and rapid communications.
- Notifiable Disease Online provides information to the public based on data from the Canadian Notifiable Disease Surveillance System. The notifiable disease charts can be used to explore trends in annual national counts and rates of reported cases of nationally notifiable diseases since 1924, where available.
- National surveillance data is routinely developed into public communication and scientific documents including, but not limited to social media, web content, infographics, publications and reports, news releases and advisories and scientific literature. Ad hoc situational reports are also developed during the response to an outbreak and often in the public domain.
- Radiation data from Health Canada's Fixed Point Surveillance Network is reported publicly in real-time through the European Union EURDEP Platform, as quarterly composites on Canada.ca, and in real-time to the International Atomic Energy Agency's IRMIS platform, which is restricted to national radiation safety authorities.
Training for surveillance activities
At the provincial and territorial level, training varies between jurisdictions as some use a mixture of university preparation, on-the-job training, orientation, residency training, and continuing education. In many jurisdictions, training in epidemiology is required for notifiable disease surveillance and clinical staff must be trained in standard operating procedures, memos, directives and communicable disease manuals and guidelines.
Public health training curricula include the essential elements of disease surveillance and reporting. The majority of training received on surveillance systems is delivered by the hiring organization (see section D4: Workforce development).
At the federal level, PHAC staff participating in real-time, infectious disease surveillance and reporting are provided with various targeted training opportunities to support these activities.
A number of programs at the National Microbiology Laboratory also provide training to external public health partners (i.e. national, provincial and regional) to support infectious disease surveillance. For example:
- PulseNet Canada and provincial clients are trained to generate, analyze and interpret genomic data for surveillance, outbreak detection and response. The National Microbiology Laboratory program provides additional training in the use of other electronic, real-time applications to support infectious disease surveillance to participating national and provincial public health stakeholders, as required, on an ongoing basis.
- The Bioinformatics Core delivers workshops and ad-hoc training sessions and working group meetings (in-person or by webinar) for use of genomic epidemiology tools used in public health surveillance (e.g. PulseNet Canada and other reference labs using genomics for surveillance). Bioinformatics Core is also in the process of developing self-directed online courseware for their workshop material. PulseNet Canada provides training to national and provincial public health partners to support their participation in national enteric disease surveillance.
PHAC's Canadian Field Epidemiology Program provides advanced training with a focus on the fundamentals and evaluation of surveillance systems, including components that build capacity for complex real-time surveillance work. These include disaster scenarios, mass gatherings, and the use of novel data to support surveillance. PHAC engages provincial and territorial partners in the development and delivery of this training. Although training is targeted to field epidemiologists, external spaces are made available to any public health professional within Canada. (See section D4: Workforce development.)
Most provinces and territories report that they have sufficient workforce capacity to perform surveillance related activities. Though the required qualifications vary across jurisdictions, most surveillance staff hold either graduate degrees in epidemiology or public health and/or have completed continuous professional education focusing on data analysis, interpretation and reporting.
Similarly, at the national level, the public health workforce has the capacity to collect, analyse, and report on data for their respective surveillance system and identify and escalate issues that would merit action. Surveillance is conducted by a variety of public health professionals including laboratory staff, epidemiologists, public health nurses, and medical officers of health (physicians).
D.2.3 Integration and analysis of surveillance data
Availability of electronic laboratory data
The integration of clinical and laboratory data is conducted sub-nationally for notifiable and other communicable diseases and then shared nationally through case-based or aggregate reporting. Generally, provinces and territories have a centralized repository or data warehouse for clinical case reporting and data identified from clinical reference microbiological laboratories. Local units report to regional health authorities, which may request further investigation (independently or on request of the province/territory).
Laboratory data are integral to national influenza surveillance connecting specific pathogens to clinical respiratory illness. The data allows further assessment of testing patterns to contextualize surveillance information, identifying genetic and antigenic properties of influenza that could influence transmission and virulence. This provides critical information that supports WHO global influenza virological surveillance, informs the composition of annual influenza vaccines, and allows for the detection of novel viruses and influenza strains with pandemic potential. This further supports national and international notification requirements.
The national influenza surveillance program collects ongoing weekly laboratory data from the National Microbiology Laboratory (strain characterization and antiviral resistance) and regional laboratories (respiratory virus detections, including influenza). Data are sent by email directly from these laboratories to the national level.
At the federal level, laboratory test requisition forms accompany the specimen when submitted for testing at local, provincial, territorial, or national laboratories. These requisition forms are standardized with limited demographic and clinical data fields. Data from the forms are entered into an electronic laboratory database. Although the data are not collected for surveillance purposes, they are linked with other epidemiologic data fields for surveillance.
Laboratory data derived nationally at the National Microbiology Laboratory are shared with the submitting provincial or territorial laboratory for clinical and public health follow-up. The data are also shared with the national influenza surveillance program lead (PHAC) for national surveillance and reporting. These data are incorporated into the weekly influenza surveillance report (FluWatch).
Where real-time, integrated electronic surveillance platforms and solutions have been adopted to support infectious disease surveillance (e.g. PulseNet enteric disease surveillance, others) both national and provincial laboratory and epidemiology stakeholders have timely access to infectious disease surveillance information to support public health investigation and response efforts. (See section D1: National laboratory system.)
Established in 2001, the Canadian Public Health Laboratory Network is a national association of public health laboratory professionals who leverage the combined strength of federal and provincial member laboratories to support efforts aimed at providing rapid and coordinated nationwide laboratory response to emerging and re-emerging communicable diseases such as SARS and pandemic influenza. (See section D1: National laboratory system.)
The Canadian Animal Health Surveillance Network is a network of federal, provincial and territorial, and university animal health diagnostic laboratories developed to improve national capacity to detect emerging animal disease threats. The Network focuses on animal disease threats with a zoonotic potential. The National Centre for Foreign Animal Disease is based in Winnipeg and links to the Network surveillance data received from multiple sources and alerts both human and animal health authorities when potential animal disease threats are identified.
In the event of a radiation emergency, radiation monitoring data from monitoring networks and laboratories is available to emergency response partners to inform situational awareness and public health protection recommendations. (See section RE: Radiation emergencies.)
D.2.4 Syndromic surveillance systems
While syndromic surveillance systems are managed at the local and provincial or territorial level, many report into national syndromic or enhanced surveillance systems. Generally, individual encounters with the healthcare system are analyzed by originating health authority. Data may be further enriched with local health and/or social services contextual data to further detect influencing factors.
Regions, provinces and territories implement syndromic surveillance systems in collaboration with the federal government as part of planned mass gatherings (Vancouver 2010 Olympics Games, Canada Games, Indigenous Games, Arctic Games, G20 Summit) as well as unplanned mass gatherings (an influx of asylum seekers in 2017).
Many jurisdictions have syndromic surveillance systems in place to offer real-time or near-time surveillance. For example:
- Alberta Real Time Syndromic Surveillance Network concurrently analyzes multiple electronic data sources in real time. Detected anomalies are immediately communicated via alerts to decision-makers for action. The Network provides integrated information on a variety of health conditions for early detection of and prompt action on abnormal events such as outbreaks and trends.
- Acute Care Enhanced Surveillance System is a near-real-time, geo-referenced emergency department syndromic surveillance system of more than 100 Ontario hospitals (both emergency department visits and admissions). The System is designed to monitor changes and trends in familiar diseases and to provide early detection of new infectious disease threats.
Canada has a variety of national syndromic surveillance systems, for example:
- FluWatch is an Influenza-like Illness Surveillance System developed to assist local and national health authorities to monitor the impact of influenza. Sentinel practitioners provide information on the presence and distribution of influenza and influenza-like illness activity across Canada.
- FluWatchers is a crowd-sourced surveillance platform that relies on volunteers to submit their experience with flu-like symptoms over a one-week period. Canadians who are not health-care professionals but who wish to contribute to influenza-like illness surveillance can also contribute via the FluWatchers online system.
- Severe acute respiratory infection surveillance consists of timely detection and reporting of unusual severe morbidity and mortality caused by both unknown and known respiratory pathogens that have the potential for large-scale epidemics or pandemics. The goal is to enable the implementation of available control measures and minimize transmission within Canada.
Severe acute respiratory infection surveillance is passive case-by-case reporting from provinces and territories to the federal government via national case report forms. Case reporting from provinces and territories to PHAC is required within 24 hours of notification to provincial and territorial health authorities. National severe acute respiratory infection surveillance was launched in 2006.
- Canadian Acute Flaccid Paralysis Surveillance System is enhanced surveillance of acute flaccid paralysis, a potential outcome of poliovirus infection. In 1994, Canada was certified as polio-free by the WHO. In 1996, Canada instituted acute flaccid paralysis surveillance to provide evidence that polio is not circulating. Cases are captured both through the Canadian Paediatric Surveillance Program and Canada's Immunization Monitoring Program ACTive (IMPACT) based in 12 pediatric-tertiary care centres. Surveillance results are reported to the Polio Global Eradication Initiative at the Pan-American Health Organization on a semi-annual basis.
Best practices, challenges, gaps and recommendations
Canada's public health surveillance system is capable of rapidly detecting public health threats, assessing risk, notifying stakeholders and the public, and responding effectively. Well-established infrastructure is in place through the PHN supported by data sharing agreements at the national, provincial and territorial levels that contributes to meeting international reporting commitments.
The geographic distribution of Canada's population in rural, remote and northern communities creates challenges in accessing health care services. In addition, basic infrastructure gaps pose barriers to national capacity for real time surveillance in some circumstances.
Data elements and data management practices have some differences across Canada as jurisdictions have lists of reportable diseases, case definitions, electronic systems, as well as privacy and security protocols that can vary in some cases. Also, diseases and conditions in certain populations can be underreported despite being captured in national surveillance; demographic characteristics remain unavailable in many surveillance systems.
Opportunities exist to leverage Canada's infrastructure to build collaborative partnerships, share information and best practices, and explore new and innovative approaches to public health surveillance. Through the pan-Canadian Public Health Network, Canada is working towards enhancing its capacity to share information, based on common data standards and establish mutual priorities.
Canada can advance technologies to increase the potential for real- or near-real time surveillance and work with data partners who have developed expertise and knowledge in providing comparable and actionable data across all jurisdictions in the country. Canada is working to expand some of the web-based systems and data sources, and develop new features, such as visual analytics and rapid risk assessment, that will be informed by a growing range of information sources. These improvements will allow for greater contextual analysis and interpretation, evidence-based decision-making capacity, early warning through continuous risk assessment and forecasting, and visualisation.
D3: Reporting
Joint external evaluation target: Timely and accurate disease reporting according to World Health Organization (WHO) requirements and consistent coordination with the Food and Agriculture Organization and the World Organization for Animal Health.
Level of capability in Canada
Canada recognizes its international obligations to report to the World Health Organization (WHO), the World Organization for Animal Health, and the International Atomic Energy Agency. Canada works to meet all reporting requirements in a timely and coordinated manner. For example, Canada's International Health Regulations (IHR) National Focal Point, at the Public Health Agency of Canada, is responsible for coordinating the implementation of the IHR on behalf of the Government of Canada. It is an IHR communications hub, accessible around the clock to the WHO IHR Regional Contact Point, relevant domestic sectors, and stakeholders for urgent communications concerning the implementation of the regulations, particularly articles 6 to 12.
Canada has performed regular IHR-related assessments and reporting using the Annex 2 decision instrument. This activity has been based both on real-life scenarios and domestic simulation exercises. Canada is also committed to timely, on-point information sharing among parties under the IHR to ensure critical public health follow-up. For more details, refer to P2: International Health Regulations coordination, communication and advocacy.
The IHR National Focal Point and technical experts at the Public Health Agency of Canada work closely with the Canadian Food Inspection Agency, which is both the designated World Organization for Animal Health contact point and the International Food Safety Authorities Network (INFOSAN) Emergency Contact Point for Canada. The Canadian Food Inspection Agency is therefore responsible for reporting certain animal health events, including zoonotic events, to the World Organization for Animal Health, and food safety issues to the WHO (INFOSAN).
The Public Health Agency of Canada, the Canadian Food Inspection Agency, and Health Canada have a joint protocol to facilitate the reporting of food safety events to the WHO through the IHR and INFOSAN networks. Similarly, Health Canada and the Public Health Agency of Canada have a joint protocol for reporting real or potential nuclear emergencies to the International Atomic Energy Agency and the WHO, under the IHR.
Indicators
D.3.1 System for efficient reporting to WHO, the Food and Agriculture Organization, and the World Organization for Animal Health
Federal contact points for international reporting
Any IHR-related reporting to WHO by Canada's IHR National Focal Point is made in collaboration with provincial and territorial partners, on behalf of the Government of Canada, which includes the following federal partners:
- Public Health Agency of Canada
- Health Canada
- Agriculture and Agri-Food Canada
- Environment and Climate Change Canada
- Canadian Food Inspection Agency
- Canada Border Services Agency
- Transport Canada
- Department of National Defence/Canadian Armed Forces
- Immigration, Refugees and Citizenship Canada
- Global Affairs Canada
- Public Safety Canada
For a full description of Canada's IHR National Focal Point, see section P2: International Health Regulations coordination, communication and advocacy.
Canada is a member of the World Health Organization for Animal Health. It fulfils its membership obligations through a designated contact point. This Delegate is housed within the Canadian Food Inspection Agency and represents Canada in the World Organization for Animal Health's World Assembly of Delegates.
There is also a technical, working-level World Organization for Animal Health contact point at the Canadian Food Inspection Agency who supports the Delegate and has a close working relationship with The Public Health Agency of Canada (including the IHR National Focal Point). The Canadian Food Inspection Agency is responsible for reporting certain animal diseases, including zoonotic events, to the World Organization for Animal Health on behalf of the Government of Canada.
The Canadian Food Inspection Agency is also the INFOSAN Emergency Contact Point for Canada, while Health Canada and the Public Health Agency of Canada are INFOSAN Focal Points. The INFOSAN Emergency Contact Point is responsible for reporting urgent food safety events and responding to verification requests from the INFOSAN Secretariat on behalf of Government of Canada, including Agriculture and Agri-Food Canada.
The Canadian Food Inspection Agency, the Public Health Agency of Canada, and Health Canada are committed to working closely on food safety issues as federal Health Portfolio partners. INFOSAN Focal Points work with the INFOSAN Emergency Contact Points on food safety events to ensure that information flows to and from INFOSAN as appropriate. The roles and responsibilities of each department in relation to food safety are further described below:
- The Canadian Food Inspection Agency delivers all food-related federal inspection and enforcement services under the authority of 13 federal acts covering all stages of the food chain and ensures that risks to food safety are reduced. The department contributes to the investigation and control of foodborne illness outbreaks through food safety investigation, testing and recall activities, and through regulatory compliance and enforcement activities. Risk assessment and appropriate risk management strategies to control affected products are based on food safety investigations. The Canadian Food Inspection Agency's Office of Food Safety and Recall is the INFOSAN Emergency Contact Point for Canada.
- Health Canada is the federal department responsible for helping Canadians maintain and improve their health. Health Canada's primary responsibilities regarding food safety are to establish standards and regulations, and respond to requests for health risk assessments on food-related hazards from the Canadian Food Inspection Agency or other stakeholders such as provincial or territorial governments. Health Canada's Bureau of Microbial Hazards is an INFOSAN Focal Point.
- The Public Health Agency of Canada's mission is to promote and protect the health of Canadians through leadership, partnership, innovation and action in public health. Among its activities is coordinating the response to multi-jurisdictional infectious disease outbreaks related to foodborne illness. The Agency's Infectious Disease Prevention and Control Branch, an INFOSAN Focal Point, leads this coordination and works closely with Canada's IHR National Focal Point--also located within the Agency.
Mechanisms for information exchange on food safety and zoonosis
Employees within the relevant federal government departments (i.e. the Public Health Agency of Canada, the Canadian Food Inspection Agency and Health Canada) work collaboratively at the technical and working levels and with provincial and territorial partners. They maintain a good exchange of information about food safety issues. One example of this strong collaboration among the three Health Portfolio partners is the joint protocol they have in place for the international reporting of food safety/foodborne illness events.Footnote 5
Zoonotic issues are handled in a similar manner. Technical experts collaborate with each other and with a strategic Director General level committee representing the federal departments involved with zoonotic diseases. The committee is tasked with: facilitating exchanges and prioritization of work, anticipating new risks, exploring opportunities for collaborative work, and facilitating joint risk assessments for events that may include a public health risk, such as avian influenza.
Training opportunities
General IHR and World Organization for Animal Health training
IHR and World Organization for Animal Health contact points receive training through formal and informal learning opportunities. This includes on-the-job training, dialogue with existing domestic and international partners and reviewing and exercising existing protocols and procedures. Key officials also take part in domestic and international meetings and workshops to expand their knowledge and encourage valuable multi-national and inter-sectoral collaboration (e.g., INFOSAN regional meetings, World Organization for Animal Health global meetings, IHR Regional meetings).
Domestic World Organization for Animal training
IHR awareness sessions are offered to relevant Canadian Food Inspection Agency employees (including World Organization for Animal Health Contact Points) as well as to Health Canada, the Public Health Agency of Canada officials and those from other relevant departments.
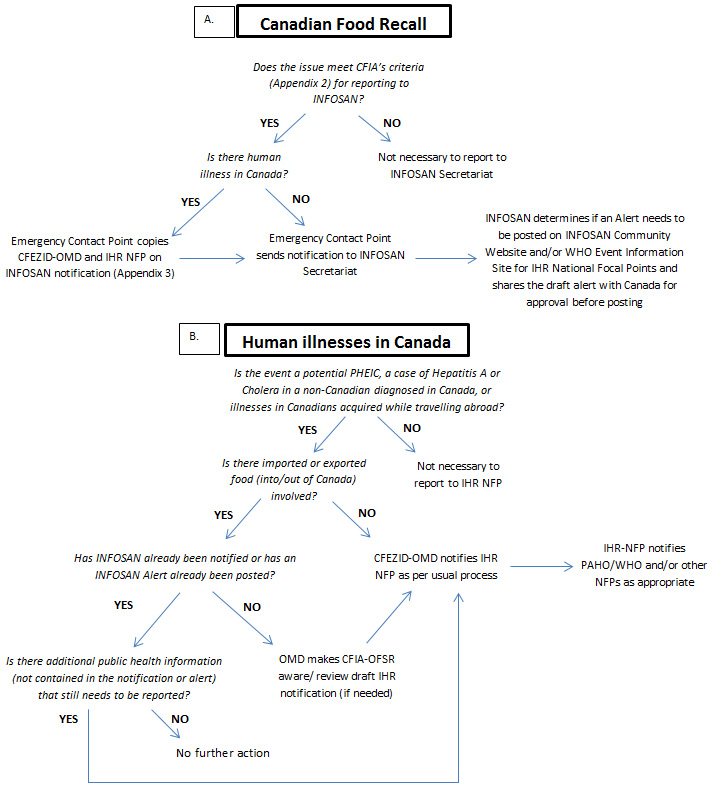
Figure 5: Flow diagram for reporting to INFOSAN and IHR network - Text description
Figure 5 is a flow diagram that outlines the process for reporting to INFOSAN and IHR network. Decision trees are used to explain the logical step by step approach in two different scenarios: A) Canadian Food Recall and B) Human Illnesses in Canada.
For a Canadian food recall, the following questions are asked:
- Does the issue meet CFIA's criteria (Appendix 2) for reporting to INFOSAN? No= Not necessary to report to INFOSAN secretariat. Yes= next question
- Is there human illness in Canada? No= Emergency Contact Point sends notification to INFOSAN secretariat.
If Yes, the Emergency Contact Point copies CFEZID-OMD and IHR NFP on INFOSAN notification (Appendix 3). As in the previous step, the Emergency Contact Point then sends notification to INFOSAN secretariat. The final step in this process is for INFOSAN to determine if an alert needs to be posted on the INFOSAN Community Website and/or WHO Event Information Site for IHR National Focal Points, and shares the draft alert with Canada for approval before posting.
For Human Illnesses in Canada, the following questions are asked:
- Is the event a potential PHEIC, a case of Hepatitis A or Cholera in a non-Canadian diagnosed in Canada, or illnesses in Canadians acquired while travelling abroad? No= Not necessary to report to IHR NFP
- Is there imported or exported food (into/out of Canada) involved? No=CFEZID-OMD notifies IHR NFP as per usual process
- Has INFOSAN already been notified or has an INFOSAN alert already been posted? No= OMD makes CFIA-OFSR aware/ review draft IHR notification (if needed), before notifying IHR NFP as per usual process
- Is there additional public health information (not contained in the notification or alert) that still needs to be reported? No= No further action
If Yes, then CFEZID-OMD notifies IHR NFP as per usual process. The final step after notifying the IHR NFP, is for the NFP to notify PAHO/WHO and/or other NFPs as appropriate.
Canada is also supportive of collaborative learning opportunities under the One Health umbrella. For example, Canada sponsored the Pan American Health Organization (PAHO) and WHO National Bridging Workshop in Costa Rica, March 2016, which brought different sectors together and promoted capacity building. The workshop was on the IHR World Organization for Animal Health Performance of Veterinary Services Pathway. The Canadian Food Inspection Agency and the Public Health Agency of Canada gave a joint presentation on Canada's experiences that illustrated the close working relationship between the two agencies.
The workshop was a useful mechanism for international collaboration and exchange between different sectors. In addition, The Canadian Food Inspection Agency participated in an antimicrobial resistance meeting hosted by the Pan American Health Organization (PAHO) and the WHO which promoted inter-sectoral collaboration.
Mechanisms for public health, animal health, and security authorities to make decisions on reporting
Public health
The IHR Annex 2 'decision instrument for the assessment and notification of events that may constitute a public health emergency of international concern' is used by decision-makers in Canada to determine whether a public health event requires reporting to the WHO under the IHR. In Canada this assessment is performed jointly by the appropriate federal technical leads, the IHR National Focal Point, the reporting province(s) or territory (ies), and other partners as necessary.
Other reporting mechanisms for decision-makers include:
- A joint protocol for the Government of Canada to report urgent food safety-related events, with clearly defined roles and responsibilities.
- Guidance on assessing and reporting chemical events under the IHR: Health Canada has developed a guidance document (currently under review) based on the IHR Annex 2 decision instrument for the assessment and reporting of chemical events.
- Reporting to the International Atomic Energy Agency: If a real or potential nuclear emergency is reported by Health Canada's Radiation Protection Bureau to the International Atomic Energy Agency in its capacity as Competent Authority for the Convention on Early Notification of a Nuclear Accident, then protocols are in place for the Radiation Protection Bureau and Canada's IHR National Focal Point to collaborate on issuing an article 6 notification to PAHO/WHO (via the IHR National Focal Point).
The content of the International Atomic Energy Agency notification, which focuses on both the technical aspect of the incident as well as protective measures, differs from an IHR notification, which focuses instead on the public health implications. (See section RE: Radiation emergencies.)
Additional reporting considerations for decision-makers include:
- Privacy considerations: Canada's Privacy Act provides the legal framework for the collection, use, disclosure, retention and disposal of personal information in the administration of programs and activities by government institutions including the Public Health Agency of Canada. The government therefore has an obligation to determine whether the information it will receive and the associated information management processes by the IHR National Focal Point complies with federal privacy requirements. Accordingly, Canada's IHR National Focal Point completed a Privacy Impact Assessment in 2016 to identify any privacy risks and related mitigation strategies. Footnote 6
- Information sharing considerations between federal, provincial and territorial partners: Developed under the Pan-Canadian Public Health Network, the Multi-lateral Information Sharing Agreement is a legal agreement that establishes standards for sharing, usage, disclosure and protection of public health information among Canadian jurisdictions for surveillance of infectious diseases and the management of pan-Canadian and multi-jurisdictional public health events and public health emergencies of international concern (see section P1.1: National legislation, policy and financing).
Public health, animal health and security
In Canada, various response plans and protocols allow for inter-sectoral collaboration between public health, animal health and security sectors to assess joint threats and to report internationally through various channels.
Informal consultation mechanisms with WHO (article 8)
Canada's IHR National Focal Point has procedures in place to use the consultation mechanism with the WHO under article 8. To date, Canada has not yet exercised this option in relation to a domestic event. The IHR National Focal Point continues to regularly consult with PAHO/WHO to seek clarification or further guidance on reports produced by PAHO/WHO, and to request further information on events or risks. For example, in 2017, Canada requested clarification/information from PAHO/WHO regarding:
- Yellow fever entry requirements in countries X and Y
- Yellow fever exit requirements
- Updates to the Zika country classification list
Overall, Canada is committed to maintaining open and transparent communication under the IHR with the WHO and often seeks clarification or further information in order to be able to contextualize regional and international guidance by PAHO/WHO for domestic partners.
Bilateral exchange mechanisms with other IHR National Focal Point
Canada is committed to timely and relevant bilateral information sharing for the purposes of critical public health follow-up in accordance with the IHR and national laws.
Use of information sharing under article 44 is especially valuable for efficiently and quickly linking technical experts in Canada with their counterparts in other countries. Examples of reports sent in 2017 by Canada to other National Focal Points under article 44 concerned:
- A case of invasive pneumococcal disease in a resident of country X
- Contacts of mumps in country X
- A case of Legionnaires' disease in a Canadian who travelled to country X
- A case of Shigella sonnei in country X
- A case of Lyme disease in a resident of country X diagnosed while in Canada
- A case of invasive meningococcal disease in Canada
- A case of measles in country X
- A case of measles in Canada with travel history in country X
- Potential exposure to measles in two country X residents
- A case of latent tuberculosis who relocated from Canada to country X
- A case of pertussis in a resident of Canada with a recent travel history to country X
- A request for information on cholera in country X
Other examples of bilateral information sharing under the IHR by Canada include:
- Information sharing with the IHR National Focal Point of country X regarding an extension to an expired Ship Sanitation Certificate (SSC) [articles 20/39]
- Information sharing with IHR National Focal Point country X regarding E. coli contamination in potable water supply onboard XYZ International Airlines aircraft in Toronto (Canada) [article 24]
- Information sharing with the IHR National Focal Point of country X related to evidence of rat infestation onboard a cargo ship [articles 39/27.2]
- Information on a traveller to country X from Canada with low risk exposure to EVD [article 30]
Information sharing takes place under the IHR via the National Focal Point network. Regular information sharing also occurs at the working level, directly between technical leads in Canada's federal Health Portfolio and their counterparts at PAHO/WHO and/or in other countries. This is the preferred method for communicating case and contact management reports and other technical (not related to Public Health Emergency Information of Concern) reports between the Public Health Agency of Canada and the United States (via the US Centers for Disease Control and Prevention).
Canada also receives information from other countries via the National Focal Point network. Some examples in 2017 concerned:
- A tuberculosis contact investigation involving air travel
- Tuberculosis cases in country X
- A case of (miliary) tuberculosis in country X
- Contacts of a case of measles on an international flight
- A case of multi-drug-resistant tuberculosis aboard an international flight
- Measles exposure in country X
- Contacts of an infectious Rubella case on a flight
- A case of dog bite to a resident of country X while visiting Canada
D.3.2 Reporting network and protocols in country
Examples of IHR event assessment and reporting
The following are two recent examples of events detected by Canada's health system that were assessed against the IHR Annex 2 decision instrument and reported to WHO. It should be noted that Canada has exercised IHR-related assessment and reporting on many occasions based both on real-life scenarios (refer to Table 5 for statistics) and during domestic simulation exercises.
| N/A | Article 6 Notification |
Article 7 Info sharing |
Article 8 Consultation |
Article 9 Other reports |
Article 10 Verification WHO requests |
|---|---|---|---|---|---|
| 2011 | 1 | 0 | 0 | 0 | 2 |
| 2012 | 2 | 0 | 0 | 0 | 2 |
| 2013 | 1 | 0 | 0 | 0 | 1 |
| 2014 | 3 | 0 | 0 | 0 | 3 |
| 2015 | 1 | 0 | 0 | 0 | 0 |
| 2016 | 5 | 1 | 0 | 1 | 1 |
| 2017 (to May 15) |
1 | 1 | 0 | 0 | 1 |
| Total | 14 | 2 | 0 | 1 | 10 |
Seoul virus (haemorrhagic fever with renal syndrome-type hantavirus) infections in Canada, possibly linked to the United States' Seoul virus outbreak associated with pet rats
On January 24, 2017, The Public Health Agency of Canada received a US CDC Health Alert via the CDC Health Alert Network of a Seoul virus outbreak associated with home-based rat breeding facilities in Wisconsin and Illinois.
On January 27, 2017, the US CDC contacted the Public Health Agency of Canada's National Microbiology Laboratory by telephone to notify of a Canadian linkage to the Seoul virus outbreak via trace back to Canadian rat breeders. The CDC also reached out directly to officials in the relevant province/territory where the rat breeders were living and operating.
The National Microbiology Laboratory conducts hantavirus reference laboratory testing for samples received from Canadian provinces and territories. This testing provides a passive surveillance system for hantaviruses in Canada. Throughout the course of this investigation, the National Microbiology Laboratory conducted testing on relevant human and rat samples.
Results were shared on an ongoing basis with the US CDC. In addition, the relevant province or territory led the investigation, with collaboration from the Public Health Agency of Canada, the provincial or territorial laboratory, the respective Ministry of Agriculture, and the affected Local Health Units.
Upon assessment, these cases of haemorrhagic fever with renal syndrome-type hantavirus infection in Canada were reported to the WHO under article 6 on February 10, 2017, concurrently with the United States. The report served to update on the U.S. investigation and notify the WHO and international community of the disease link to Canada. This notification was developed collectively by The Public Health Agency of Canada's technical experts and their collaborating provincial and territorial partners, and approved by senior officials from their respective organizations.
Case of Vibrio cholerae in Canada
On April 24, 2017, a single laboratory confirmed case of Vibrio cholerae O1, Biotype El Tor, Serotype Inaba was identified in Canada. Because the case did not report a history of travel outside of Canada, the event was assessed using the IHR Annex 2 Decision Instrument as a potential Public Health Emergency of International Concern.
Vibrio cholerae is a notifiable disease in Canada. When the pathogen was detected through laboratory testing, the case was reported to the appropriate Medical Officer of Health in accordance with provincial and territorial legislation. In turn, the case was reported to the National Enteric Surveillance Program and subtyping (pulsed field gel electrophoresis and whole genome sequencing) was conducted by PulseNet Canada. Laboratory and public health surveillance systems functioned seamlessly in the identification of this health event.
Federal and provincial public health decision-makers and leadership collaborated on assessing the event and reporting it to PAHO/WHO. Appropriate individuals were consulted and informed in accordance with existing protocols within the Public Health Agency of Canada. Informal information exchange among technical staff was also critical to the decision-making process.
Upon assessment, this case of Vibrio cholerae was reported to PAHO/WHO under article 7 (Information sharing during unexpected or unusual public health events) on May 10, 2017. Federal and provincial Ministries of Health were engaged in the event.
IHR assessment and reporting process
Canada has guidelines, procedures, and tools in place to report to the WHO under the IHR. Any urgent public health event must be reported by a province or territory or other source to the Public Health Agency of Canada as the IHR National Focal Point. In accordance with the IHR, Canada then has up to 48 hours to complete an assessment based on the IHR Annex 2 decision instrument. This is done in collaboration with the reporting province or territory, Canada's IHR National Focal Point, and other relevant partners.
Once the technical experts determine that an event may constitute a Public Health Emergency of International Concern, they have up to 24 hours to notify the WHO and initiate any health measure implemented in response to those events. If the event does not meet the criteria for notification under article 6 of the IHR, then other IHR-related requirements for reporting to WHO and/or other States Parties may still apply. These may include those under article 7 (information sharing during unexpected or unusual public health events), article 9 (any public health risk that may cause international disease spread), or article 44 (collaboration and assistance).
Key parties involved in the preparation and approval process for article 6 notifications:
- The National Focal Point coordinates the IHR reporting process, provides policy advice with regard to the assessment and reporting processes, and maintains overall situational awareness of any potential IHR notifications. The National Focal Point assigns timelines to contributors and approvers so that Canada meets its reporting obligations timelines under the IHR; and it shepherds the draft notification through senior management approvals. Once PHAC's President and the Chief Public Health Officer of Canada have approved, the National Focal Point sends the notification to PAHO/WHO and other relevant domestic and international partners.
- The IHR technical advisor is required to remain available to provide IHR technical advice with regard to completing the IHR assessment using the Annex 2 decision instrument, and to assist with drafting the article 6 notification.
- Health Portfolio technical leads are required to complete the article 6 notification template and any related bilateral IHR information sharing notices (i.e. article 44) within assigned timelines, in collaboration with the reporting province/territory, IHR Technical Advisor and other appropriate federal and international partners. They must also obtain approvals at the program-level (up to the Branch Assistant Deputy Minister or Vice President) and from the relevant provinces or territories (up to the Chief Medical Officer of Health).
- As the key decision-makers, Senior Management (Health Portfolio) is required to review and approve draft notifications within specific timelines. Senior Management is also responsible for briefing other federal partners, if applicable.
- Provincial and territorial partners are required to provide relevant public health information in a timely manner in order to complete the reporting template jointly with Health Portfolio technical leads, and to approve the draft notification within specific timelines.
It should be noted that, if the Health Portfolio Operations Centre is activated to support the event, then additional officials from the incident management system (i.e. operations team, operations chief, emergency/event manager) will play a role in the IHR reporting and approval process.
In addition, the Health Portfolio Operations Centre Watch Office has its own internal processes to support the IHR assessment and reporting process (see section P2: International Health Regulations coordination, communication and advocacy).
Best practices, challenges, gaps and recommendations
Information sharing in support of timely reporting is an area for ongoing collaboration across different levels of government in Canada. For example, the Zika virus is not yet listed as requiring notification from public health to federal authorities given its recent emergence. Some provinces and territories have noted difficulty finding the appropriate authority at the national level to report cases to during and after the declaration of a Public Health Emergency of International Concern by the WHO. However, the federal government will almost always coordinate a PHEIC within the Health Portfolio; therefore, any related data would be immediately forwarded to Canada's IHR National Focal Point within PHAC.
Further, the reporting requirements from WHO for Zika virus and related complications were at times complex, which made it challenging for officials at the federal, provincial and territorial levels of government to coordinate information sharing and reporting activities. As a result, technical leads at all government levels collaborated to negotiate terms for information sharing and were able to provide as much information as possible to meet WHO reporting requirements.
This exercise also prompted provinces and territories to examine their information sharing authorities and, at the national level, a review of the list of notifiable diseases. Additional efforts are required to further streamline and facilitate information sharing across jurisdictions to ensure effective and efficient reporting.
A best practice for radiation emergencies is coordination between the IHR National Focal Point and the Competent Authority for the International Atomic Energy Agency (Health Canada) to harmonize emergency notifications and reporting to both the International Atomic Energy Agency and WHO.
It is recommended that the Public Health Agency of Canada (technical lead and the IHR National Focal Point) work closely with the Canadian Food Inspection Agency to explore joint protocol options for the international reporting of zoonosis-related events to the WHO, the World Organization for Animal Health and the Food and Agriculture Organization of the United Nations. It would resemble Canada's joint protocol for reporting food safety/foodborne illness events to WHO and INFOSAN.
In order to further strengthen assessment and reporting in Canada, the IHR National Focal Point is exploring new and innovative ways to promote the IHR and to provide valuable learning opportunities for stakeholders. Prioritizing a flexible approach tailored to specific audiences and individual needs, the IHR National Focal Point's new outreach and training strategy aims to bolster its virtual and web offerings, while maintaining a critical face-to-face presence. While emphasis in recent years has been on federal-level partners, work is underway to enhance engagement with provincial and territorial partners.
D4: Workforce development
Joint external evaluation target: State Parties should have skilled and competent health personnel for sustainable and functional public health surveillance and response at all levels of the health system and the effective implementation of the International Health Regulations (2005).
Level of capability in Canada
Canada has made significant progress developing its public health workforce capacity over the past 15 to 20 years. Federal, provincial and territorial jurisdictions have worked to equip Canada with a competent and capable public health workforce to carry out public health surveillance and response as required under the International Health Regulations. Federal, provincial and territorial public health organizations currently employ a diversified workforce that includes:
- Epidemiologists
- Public health advisors
- Physicians (public health and preventive medicine, infectious disease specialists, and other specialties)
- Nurses (community health and infection prevention and control)
- Surveillance analysts
- Veterinarians
- Laboratory specialists and laboratory technicians
- Environmental health officers/public health inspectors
- Information system and technology specialists
- Public health communications specialists
Canada has the ability to mobilize public health staff anywhere within the country to complement provincial and territorial public health response efforts. Certain jurisdictions have reported shortages in public health professionals with adequate skills. Due to Canada's size and geography, these challenges will continue to exist, especially in rural, remote, northern, and Indigenous communities. However, public health professionals from other jurisdictions (regional, provincial, territorial, and federal) are available on request to support the response to a public health threat in affected jurisdictions.
Public health training for various disciplines in Canada is delivered in a variety of ways through universities, various levels of government and non-governmental organizations (NGOs). Master's and/or Doctoral degree programs in epidemiology are offered at several universities throughout Canada. The Canadian Field Epidemiology Program provides advanced training for field epidemiology.
Indicators
D.4.1 Human resources are available to implement IHR core capacity requirements
Federal public health professionals
The Public Health Agency of Canada (PHAC) leads public health surveillance, emergency preparedness and response to all-hazards public health events and threats. The PHAC workforce consists of epidemiologists, clinicians, nurses, surveillance analysts, laboratory technicians and social scientists. Health Canada and Indigenous Services Canada, which provide services to specific populations such as First Nations on reserve and military personnel, employ physicians, nurses, environmental public health officers, epidemiologists, pharmacy personnel and dental professionals.
Although there are some veterinarians employed within PHAC, Health Canada and Environment and Climate Change Canada, the majority are employed by other ministries such as the Canadian Food Inspection Agency and Agriculture and Agri-Foods Canada at the federal level.
Information specialists are typically employed through Shared Services Canada.
The Department of National Defense has sufficient human resources to assist in the event of a national public health emergency. The Canadian Armed Forces has a variety of public health trained providers. The Forces' preventive medicine technicians serve as public health inspector equivalents. Each base or wing has a senior physician in charge of overseeing all matters related to public health and to liaise with the civilian public health authorities and the Forces public health authority. Overall there are approximately 60 trained specialists covering a wide range of disciplines.
For responding to radiation emergencies, the workforces of Health Canada and its federal emergency response partners, including PHAC, the Canadian Nuclear Safety Commission, Natural Resources Canada, and Environment and Climate Change Canada, consist of trained and experienced scientists, technologists and other professionals who will contribute to a response.
Intermediate and regional public health professionals
Provinces and territories possess full or partial capacity to meet and implement IHR core capacity requirements within the scope of their practice. In Canada, these jurisdictions all have epidemiology, laboratory, and case management and information system specialists. In some provinces and territories these resources may be centralized, while in others they may be spread over multiple departments or agencies-with associated advantages and disadvantages for mobilization.
Some provinces (British Columbia, Ontario, Quebec) have public health agencies separate from provincial government departments to provide specialized scientific, technical, and laboratory functions.
Smaller population bases (rural, remote and Indigenous) have historically reported challenges in recruiting and retaining core and specialized public health staffFootnote 7.
Capacity across the country by public health discipline group
Canada possesses a wealth of skills in its public health human resources, although capacity is not evenly distributed amongst discipline groups or geographic areas. However, there are mutual aid agreements in place to facilitate the sharing of resources when required.
Epidemiology and field epidemiology
While in some provinces and territories, the resource capacity is quite limited, every jurisdiction has access to trained epidemiologists. They are often dedicated to specific areas such as communicable disease or immunization. For instance, epidemiologists are available in all local public health units in Ontario and at the provincial level. Epidemiologists are typically involved with surveillance, response, and preparedness.
In terms of field epidemiologists, Canada has sufficient capacity to support investigations throughout the country. Epidemiology support can also be provided to provinces and territories through two federal programs:
-
Canadian Public Health Service places public health officers (experienced public health practitioners from a variety of professional disciplines) with provincial, territorial and local health authorities, and non-governmental organizations to address both ongoing and emerging needs. These two-year placements benefit the host organization and strengthen Canada's public health workforce and professional network.
The Canadian Public Health Service program helps build pan-Canadian, public health emergency management capacity by placing qualified public health officers in federal, provincial and territorial public health organizations that are under-resourced. This is a flexible and scalable workforce that can be used for surge capacity when PHAC needs to respond to a public health event. There are currently 16 public health officers available for deployment.
-
Canadian Field Epidemiology Program trains public health professionals (nurses, physicians, epidemiologists, etc.) in applied epidemiology. This consists of specialized techniques and competencies required to respond to diverse public health issues in a variety of settings.
At any point in time, the Program has 10 active field epidemiologists ready to be deployed anywhere they are needed within Canada, supporting public health organizations as they respond to urgent public health events. They constitute PHAC's first line of field epidemiology surge response.
Graduates of the Program are employed at PHAC, provincial, territorial, and local health authorities, as well as at non-governmental organizations, forming an important collaborative network of public health practitioners. While Program alumni are not present in all provinces and territories of Canada, hundreds of public health professionals, mostly epidemiologists have attended Program sponsored applied epidemiology training.
Physicians
Physicians must be licensed to practice medicine in a province or territory in Canada in order to be eligible for employment as a medical officer or medical specialist at the national or intermediate level. All active physicians in Canada must participate in continuing medical education as part of annual registration requirements.
At the federal level there are currently PHAC medical officers and specialists available to respond within the Agency to a range of routine activities related to protecting the health of Canadians. They are available during public health emergencies to provide subject matter expertise, public health advice, and to conduct risk assessments. They can also act as liaisons to other federal departments or to the provinces and territories during public health emergencies. The majority of provinces and territories have existing capacity within their jurisdiction, but this can vary by site, specialty and/or geographical location.
Nursing
Defined nursing positions require a license to practise nursing in a province or territory in Canada. PHAC nurses are available to respond within the Agency during routine activities within the Agency's mandate, and during public health emergencies (providing subject matter expertise and public health advice, developing guidelines, etc.). They can also act as liaisons to other federal departments or to the provinces and territories during public health emergencies.
Indigenous Services Canada employs community health nurses to provide basic primary care and public health to First Nations communities on reserve-these nurses are all trained in key primary care and public health competencies.
There are many licensed nurses in non-nursing positions, such as epidemiologist or policy analyst classifications. At the provincial and territorial level, most jurisdictions have the capacity to meet the
IHR requirements with respect to this public health discipline.
Laboratory specialists and technicians
PHAC's National Microbiology Laboratory is Canada's main infectious disease public health laboratory with responsibility for:
- Reference microbiology and quality assurance
- Laboratory surveillance for infectious diseases
- Emergency outbreak preparedness and response
- Training
- Research and development
The National Microbiology Laboratory is equipped with laboratories ranging from biosafety level 2 to level 4. The National Microbiology Laboratory Special Pathogens Program and the Bio-forensics Assay Development and Diagnostics Section are mandated with providing laboratory capacity (diagnostic capability) for national public health emergency response mobilizations. National Microbiology Laboratory laboratories and provincial and territorial laboratories work collaboratively, and PHAC provides laboratory liaison officers to assist in meeting provincial and territorial requirements where possible.
Health Canada's Radiation Protection programs contain significant laboratory capacity and expertise for responding to a radiation emergency (environmental and human radiation monitoring, analysis, and assessment).
Information specialists and assistants
At the federal level, information system specialists are available to other federal departments through an agreement with Shared Services Canada. This is the federal organization that provides information system/information technology support to all federal departments and agencies. These employees generally have diplomas or bachelor degrees in information systems and technology or computer science. This community has a strong culture of professional development and continuing education due to the ever-changing field of technology.
The majority of province/territories reported having access to information system specialists at arm's length to support all health-related electronic systems, extract datasets for analysis, integrate data from some databases, and generate reports. However, certain provinces did indicate that their capacity may not extend to certain sites/geographical locations.
Social scientists
At the federal level, PHAC has a range of Masters and PhD prepared social scientists on staff. These employees, like other PHAC staff, have access to professional development activities supported by the Government of Canada policy on learning, training and professional development for government employees. At the provincial and territorial level, the majority of jurisdictions employ social scientists and they are able to meet and implement core capacity requirement within their scope of practice.
Surveillance analysts
PHAC and Indigenous Services Canada have a wide range of surveillance analysts whose main tasks are related to:
- The tracking and forecasting of any health events, from chronic diseases to infectious diseases to health determinants, through the ongoing collection of data or open source information
- The integration, analysis and interpretation of data/information into surveillance products
- The dissemination of surveillance products to federal, provincial and territorial levels in order to undertake necessary actions or responses
Veterinarians and veterinary technicians
At the federal level, veterinarian capacity resides in departments including the Canadian Food Inspection Agency, Agriculture and Agri-foods Canada and Environment and Climate Change Canada. PHAC veterinarians work as technical advisors or epidemiologists in the Infectious Disease Prevention and Control Branch, or at the National Microbiology Laboratory's Guelph and Saint-Hyacinthe locations. Provinces and territories have some veterinarian capacity at the intermediate level. (See section P4: Zoonotic disease for more information on veterinary and animal health workforce.)
Public health inspectors and environmental health officers
Public health inspectors and environmental health officers, working in all levels of government, must be certified through the Canadian Institute of Public Health Inspectors in order to practise in Canada. At the federal level, environmental health officers, like all regular members of the Institute, participate annually in the Institute's Continuing Professional Competencies Program. Members self-assess against their discipline-specific competencies and set competency-based learning objectives. They collect and submit professional development hours to the Institute as part of maintenance of certification.
Other relevant public health personnel
Provinces and territories also employ a variety of additional public health personnel, including: entomologists, nutritionists, biostatisticians, communications specialists, health educators, and emergency preparedness specialists
In the North, there are also many community-based health and social service workers (e.g. community health representatives) who play a key role in the northern public health workforce.
D.4.2 Field Epidemiology Training Program or other applied epidemiology training program in place
Field Epidemiology Training Program
There is a single national program of advanced field epidemiology training delivered by the federal government. Created in 1975, Canadian Field Epidemiology Program is one of only three field epidemiology training programs in the world, along with the U.S. Epidemic Intelligence Service and the U.K. Field Epidemiology Training Programme, which has been accredited by the advisory board of the Training Programs in Epidemiology and Public Health Interventions Network, which recognizes the excellence in field epidemiology training.
PHAC public health training experts develop the applied epidemiology training delivered to trainees under the Training Programs in Epidemiology and Public Health Interventions Network Canadian Field Epidemiology Program. The Program has graduated 187 epidemiologists in the last 43 years and involves collaborations with various domestic and international partners, including:
- The WHO's Global Outbreak Alert and Response Network
- The European Centre for Disease Prevention and Control's field epidemiology path and the European Programme for Intervention Epidemiology Training
During their two-year program, field epidemiologists participate in training activities in various formats including virtual or online learning (1 week) and in-person classroom training (8 to 10 weeks in total). Training modules are regularly updated and modified to make sure field epidemiologists have the necessary skills and competencies to respond to emerging threats. Training includes:
- Outbreak investigation and management
- Surveillance (evaluation, mass gathering, etc.)
- Advanced applied epidemiology (time-series analysis, social network analysis, spatial epidemiology, etc.)
- Scientific competencies (scientific writing, presentation skills, risk communication, etc.)
- Site placements with appropriate surveillance projects, mentorship and experience
In-kind training is also offered in-kind to epidemiologists working in public health across the country (federal, provincial or local level). For every Canadian Field Epidemiology Program trainee attending the training, three external epidemiologists are trained.
Canada has established agreements with international organizations to share field epidemiologists during emergency events. For example, during the Ebola outbreak in West Africa, PHAC had mobilized field epidemiologists and Canadian Field Epidemiology Program alumni who, together, provided epidemiological support in Guinea and Sierra Leone. The Canadian Field Epidemiology Program also supported the TEPHINET Step initiative in Senegal to help prepare the country for an Ebola response.
Examples of other public and private sector public health training
Additional training in public health is available to various public health discipline groups during and after formal academic preparation, including:
-
In epidemiology, master's and/or doctoral level programs are offered at numerous universities in British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Quebec, Newfoundland (clinical epidemiology), Nova Scotia and Prince Edward Island. Some programs offer joint degrees in biostatistics.
Graduate programs in epidemiology tend to focus on research rather than applied epidemiology and are mainly thesis based. Some universities offer a Master of Health Sciences degree which has a strong epidemiology and biostatistics curriculum.
Students in these programs may opt for a thesis track or a practicum experience track. Students in the latter track are placed in work settings, such as public health organizations, to gain work experience prior to graduation.
-
For physicians, currently, there are 13, five-year, Royal College of Physicians and Surgeons of Canada Public Health and Preventive Medicine Residency Programs in Canada at universities in British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, and Quebec.
Residents may undertake rotations at the local, regional, provincial, territorial and federal levels of public health. The medical school curriculum provides opportunities for public health elective rotations (anywhere from two to eight weeks). Medical students can apply to a five-year public health and preventive medicine residency program.
Physicians may earn a Master of Public Health or a master's or doctoral degree in relevant public health fields (e.g. epidemiology). Physicians may also pursue certificates relevant to public health practice (e.g. in epidemiology and biostatistics) through many universities.
-
Entry to practice nursing in Canada requires a bachelor's degree. Nursing students can undertake their fourth year integrated practicum in a public health setting. Master's level nursing programs allow for specialization in public health. Nurses may pursue a Master of Public Health or a master's or PhD in relevant public health fields (e.g., epidemiology).
Nurses may also pursue certificates relevant to public health practice (in epidemiology, health promotion, and biostatistics) through many universities. The Canadian Nursing Association offers RN certification in community health, among other nursing practice specialties. The Community Health Nurses of Canada was created in 1987 to provide support to this sub-specialty of nursing.
-
In biostatistics, there are master's and/or doctoral degree programs offered at numerous universities in British Columbia, Alberta, Saskatchewan, Ontario, and Quebec. They are all thesis-based programs. Many graduate programs in health sciences and epidemiology offer concentrations in biostatistics.
The federal government oversees a research affiliate program that permits graduate and doctoral students in biostatistics to work at PHAC and undertake projects with federal epidemiologists or biostatisticians. The program supports recruitment into PHAC or other federal departments after graduation.
-
Universities across Canada offer bachelor's and master's degree programs in information systems and technology. Many are co-operative education programs, meaning that students undertake placements in a work setting to gain practical, hands-on experience prior to graduation.
Diploma programs are also offered through many Canadian colleges. These are practical training programs, grounded in real world experience for students. It is unclear to what extent practicum placements occur in public health organizations or if public health settings are actively recruited to serve as practicum placements site.
- Surveillance analysts may have taken bachelor's, master's, and doctoral degree programs in a variety of fields, such as epidemiology, biology, biostatistics, medical or nursing sciences, social sciences, which are offered at universities throughout Canada. Graduate programs are generally thesis based.
- Canada has five veterinary colleges located at universities in Prince Edward Island, Quebec, Ontario, Saskatchewan and Alberta.
- There are many bachelor's, master's, and doctoral degree programs in social sciences offered at universities throughout Canada. Graduate programs are generally thesis based.
- Practical medical laboratory technologist/technician diploma programs-with curricula grounded in real-world experience-are offered at many Canadian colleges.
- Universities across Canada offer bachelor's, master's, and doctoral degree programs in biology and related laboratory fields. These programs are thesis based.
- In order to effectively implement the IHR, a basic understanding of emergency management principles is required to ensure health considerations are properly reflected in planning, preparedness and response activities. Several emergency management programs exist at universities and colleges in Canada, including York University, Ryerson University, George Brown College, and the Justice Institute of British Columbia. PHAC is developing a baseline generic emergency management training program for all employees interested in supporting emergency response activities.
- To ensure the health workforce is equipped to meet emerging health challenges, the Health Portfolio has developed just-in-time online training. For example, in 2015 when Canada announced the project to welcome 25,000 Syrian refugees to Canada, PHAC developed a series of webinars for health professionals on delivering mental health services to the refugees.
There is currently no comprehensive tracking of graduates from the programs offered by Canadian universities and colleges. Consequently, Canada does not have data on the number of graduates of these programs working in government and non-government public health organizations.
D.4.3 Workforce strategy
In 2005, Canada produced Building the Public Health Workforce for the 21st Century: A Pan-Canadian Framework for Public Health Human Resources Planning. Some elements of this framework were addressed, including development of core competencies for public health to help inform interdisciplinary approaches to defining workforce capacity and to guide workforce development. Implementation and application of this model varies across and within jurisdictions.
Provincial and territorial public health departments or agencies, and Indigenous organizations, have workforce strategies aligned with the priorities of their respective jurisdictions. These can be either proactive and tailored to population health priorities or responsive in nature. Many local public health organizations also have their own internal workforce strategies. These jurisdictional workforce strategies are used to guide staffing, development, and retention of their respective workforces.
For instance in British Columbia, BC's Guiding Framework for Public Health establishes a long-term vision and a set of goals for the public health system. The workforce is identified as a foundational element to contributing to the program elements of the public health system (i.e. health improvement; prevention of disease, injury and illness; environmental health; public health emergency management). The strategy notes that there will be an ongoing focus on public health human resource training and development.
Provincial and territorial workforce strategies exist in varying degrees of formality and stages of implementation, and some are being revisited due to restructuring within the jurisdiction. Greater alignment is required for more coherent assessment and planning of Canada's overall workforce capacity.
At the federal level, PHAC approved an internal workforce development strategy, the Building Capacity and Investing in People: Public Health Workforce Development Strategic Plan 2015-2019. Elements of the plan are being applied to guide workforce capacity assessment and development to respond to public health events.
Available data for the health workforce suggest that the proportion of workers eligible to retire in three to five years is in the range of ten to 20%, if the public health workforce is comparable to the health workforce.Footnote 8
Financing the public health workforce
Public health in Canada is funded at both the provincial and federal levels. The financing of health care is provided via taxation both from personal and corporate income taxes, as well as other federal and provincial revenues.
At a federal level, funds are allocated to provinces and territories via the Canadian Health and Social Transfer. Transfer payments are made as a combination of tax transfers and cash contributions. Provinces and territories are responsible for how they budget for their public health workforce.
Best practices, challenges, gaps and recommendations
A best practice in public health workforce development is illustrated by the 2007 national (federal, provincial and territorial) initiative that developed the Core Competencies for Public Health in Canada, a framework for defining and assessing workforce capacity across disciplines and jurisdictions both within and outside of the traditional public health workforce.
The Core Competencies are being used to varying extent by approximately 50% of Canadian public health organizations to inform their workforce development plans, including employee development, recruitment, and retention. In remote areas of the country there is an on-going need to expand training opportunities for community health workers.
The primary gap and challenge is the lack of meaningful data on the composition, numbers and capabilities of the public health workforce across Canada. There have been efforts to address gaps in public health education and training, which include:
- Expanding population and public health content for some professions to meet the demands of public health practice.
- Establishing a public health and preventative residency program at the Northern Ontario School of Medicine (with the goal of expanding the pipeline of public health physicians trained to serve rural, remote and indigenous populations).
- Expanding inter-professional education programs.
- Creating teaching public health units and implementing other strategies to better connect academia and practice.Footnote 9 A meeting of the Network of Schools and Programs in Public Health in March 2016 identified the benefits of the schools working with employers to ensure that graduates of their programs (primarily Masters of Public Health) are prepared for the realities of the workplace.Footnote 10
R1: Preparedness
Joint external evaluation target: Preparedness includes the development and maintenance of national, intermediate and community/primary response level public health emergency response plans for relevant biological, chemical, radiological and nuclear hazards. This covers mapping of potential hazards, identification and maintenance of available resources, including national stockpiles, and the capacity to support operations at the intermediate and community/primary response levels during a public health emergency.
Level of capability in Canada
Emergency management in Canada is a responsibility shared across many stakeholders at municipal, provincial and territorial, and federal levels. Emergency management planning in Canada is predicated on the existence of a series of established and approved frameworks, systems, and plans.
As required by legislation, all jurisdictions have plans that set out the steps to be taken in the event of an emergency or disaster. Jurisdictions identify linkages and channels of communication to international and national level health organizations, other ministries, programs and agencies of the Government of Canada. These plans are linked, but each can also be used as a stand-alone document that contributes to a coordinated, system-wide approach to emergency management.
In Canada, there are multiple national, regional, provincial and locally run exercises that take place each year. Exercises range from small workshops and tabletop exercises to large, complex, multi-jurisdictional exercises. In general, exercises are used to mitigate risks by:
- Demonstrating due diligence and compliance with legislation, regulation, and policy
- Assessing and increasing the readiness of people and response organizations, as well as plans, policies, procedures, technology, and other resources
- Identifying internal and external system strengths, and opportunities for improvement
- Preparing and testing risk treatments and preparations to respond to a specific hazard, event or emergency
Many federal, provincial and territorial governments engage in risk-mapping exercises through processes customized to their jurisdictions. This mapping considers both geographical risks and population characteristics. Risk assessment processes are leveraged to inform strategic emergency management planning across the country, including the development of specific emergency response plans and the acquisition of medical countermeasures when appropriate.
Federal, provincial and territorial governments collaborate to ensure stockpiles of relevant supplies and medical countermeasures are maintained and available for rapid deployment during emergencies. Mechanisms have also been negotiated to rapidly mobilize health professionals during emergencies.
During emergencies different levels of government (municipal, provincial/territorial, and federal) also work in close collaboration with partners, including the Canadian Red Cross, who maintain rosters of qualified volunteers and support emergency response activities, including providing emergency social services.
Indicators
R.1.1 Multi-hazard national public health emergency preparedness and response plan is developed and implemented
Frameworks
The Emergency Management Framework for Canada is the cornerstone framework of emergency management in Canada and endorsed by all jurisdictions. It encompasses the four interdependent but integrated components of emergency management: prevention/mitigation, preparedness, response, and recovery.
The document describes the components, principles, governance, and coordinating instruments for emergency management in Canada. It is in accordance with this framework that emergency management is based on an "all hazards" approach in Canada. It addresses federal, provincial, and territorial vulnerabilities from both natural and human-induced hazards.
Parallel to this framework is the National Framework for Health Emergency Management. It was developed by the Canadian health sector and endorsed by the national Conference of Deputy Ministers of Health. The Framework is a comprehensive, integrated guiding document for health emergency management in Canada. Its goal is to establish principles and elements that will provide a context for leadership and coordination through federal, provincial and territorial emergency management systems in the Canadian health sector.
Plans
Legislation requires all jurisdictions in Canada to have plans setting out the steps to be taken in the event of an emergency. These plans identify linkages and channels of communication to other ministries, programs, and agencies of the Government. They are required to create a coordinated, system-wide approach to emergency management.
Federal, provincial and territorial health sector plans
In 2017, all jurisdictions in Canada approved the Federal, Provincial and Territorial Public Health Response Plan for Biological Events. The plan outlines how the national response to public health events caused by biological agents will be conducted and coordinated. It focuses on the implementation of responses led by senior-level federal, provincial, and territorial public health decision-makers. Improving effective engagement among public health, health care delivery, and health emergency management authorities is a key objective of the plan, which includes:
- The roles, responsibilities and authorities of federal, provincial and territorial governments for public health and emergency management
- An outline of four scalable response levels and a governance structure designed to facilitate an efficient, timely, evidence-informed and consistent approach across jurisdictions
In addition to this overarching plan for the health sector, the Canadian health sector has well-established, national hazard-specific tools. These are routinely used for effective planning and management of public health events. Some examples are Canadian Pandemic Influenza Preparedness: Planning Guidance for the Health Sector, and Food-borne Illness Outbreak Response Protocol.
A feature of the Federal, Provincial and Territorial Public Health Response Plan for Biological Events is that it is intended to complement, and where appropriate, be used in conjunction with these existing mechanisms. For example, Canadian Pandemic Influenza Preparedness: Planning Guidance for the Health Sector provides pan-Canadian planning guidance for pandemic influenza. In a pandemic, it is expected this tool will inform the technical aspects of the response while the Federal, Provincial and Territorial Public Health Response Plan for Biological Events will provide the overall governance structure that will support multi-level government decision-making.
Provincial and territorial plans
Provinces and territories have primary responsibility for delivery of health care in Canada. They maintain their own response plans, which describe emergency response governance, linkages and channels of communication between ministries, programs and agencies of government, non-governmental organizations and the private sector. Indigenous Services Canada is responsible for services in certain remote First Nations communities. However, the needs of these communities are taken into consideration in most provincial and territorial emergency response plans.
All provincial and territorial governments have plans and procedures in place to respond to emergencies with health consequences. Each plan reflects the geographical risk profiles and population needs of that province or territory. These may include hazard-specific plans, as well as standard operating procedures and memoranda of understanding to mobilize human resources and assets as required. Each province and territory has its own governance and response structures for coordinating a response. For example:
- British Columbia maintains provincial hazard-specific plans for nuclear response, pandemics, wildfire smoke, and foodborne illness outbreaks, as well as:
- An all hazard concept of operations
- A provincial Comprehensive Emergency Response Plan
- Health Emergency Coordination Centre Standard Operating Procedures
- Health authority facility and program-level emergency plans
- Saskatchewan is a member of the Pacific Northwest Border Health Alliance and is developing policies and procedures for support during emergencies. The Saskatchewan regional health authorities have a memorandum of understanding for support in emergency and can also share equipment and personnel within the province.
- The Northwest Territories maintains the following plans and equipment:
- Health and Social Services all hazards emergency response plan
- Pandemic plan
- Business continuity plan
- Template for relocation of a health centre
- Mini-clinic in Yellowknife that can be relocated during an emergency.
Federal plans
At the federal level, all departmental response plans must be aligned with Public Safety Canada's Federal Emergency Response Plan (FERP). This is the Government of Canada's all-hazards response plan, which describes the roles and responsibilities of federal institutions in an emergency. It includes a description of the "whole of government" governance framework.
The FERP provides the federal framework for emergency responses that require a centralized Government of Canada approach. It applies to domestic emergencies and to international emergencies with a domestic impact. Health Portfolio response plans and governance are aligned with the FERP.
The FERP outlines how the federal response will be coordinated through two response systems:
- The Federal Emergency Response Management System is a comprehensive emergency management system which links national and regional levels of activity. It creates both vertical and horizontal integration of effort within the federal government and integrates federal emergency response actions with those of the provinces and territories.
- The National Emergency Response System helps harmonize joint federal, provincial, and territorial responses to emergencies. It supports and facilitates procurement and logistics coordination among all levels of government, the private sector, non-governmental organizations and international stakeholders.
Both response systems have emergency management systems and governance mechanisms in place. These tools support the federal leadership in making timely decisions, coordinating activities and resources at a strategic level, and communicating effectively with federal entities, provinces and territories, international organizations, NGOs, the private sector and the public.
Terrorism plans
The Federal Terrorism Response Plan is the Government of Canada's guidance document for planning security and intelligence actions. Approved by federal Ministers, the plan aims to strengthen coordination among security and intelligence agencies and all levels of federal government by bringing them together under one overarching plan for responding to terrorism.
It is designed to address domestic terrorist incidents, as well as acts of terrorism committed abroad that implicate domestic security and intelligence agencies. Its focus is on the security and intelligence response and its links to the crisis response and consequence management components coordinated under the FERP. (See section R3: Linking public health and security.)
Federal Health Portfolio plans
In accordance with the Federal Policy for Emergency Management, the Health Portfolio maintains the Health Portfolio Strategic Emergency Management Plan. The Plan provides strategic guidance for emergency management activities across the portfolio and describes broad objectives and roles and responsibilities.
The Plan encompasses an all hazards, risk-based approach to emergency prevention, mitigation, preparedness, response, and recovery pertinent to the Health Portfolio's mandate, resources, and services. It establishes its governance structure for mitigation, preparedness, and response to emergencies.
The Health Portfolio Strategic Emergency Management Plan is accompanied by the Health Portfolio Emergency Response Plan, which is the Portfolio's all-hazards response plan. It provides guidance when the scope and/or intensity of an event goes beyond normal program level operations and necessitates a coordinated Health Portfolio response.
It provides information on how the response is coordinated throughout the portfolio and contains the operational guidance and procedural tools applicable to an all-hazards response. The Health Portfolio Emergency Response Plan is linked to parallel plans for each of the Public Health Agency of Canada's regions and supported by additional event or hazard-specific annexes for:
- Chemical emergency response
- Nuclear emergency response
- Chemical, biological, radiological, nuclear and explosives (CBRNE) intentional events
- First Nations on-reserve
- Risk communications
Parallel plans are linked within each region of PHAC.
The IHR core capacity requirements are covered within the Health Portfolio Emergency Response Plan. It outlines an action plan for ensuring that the core capacities, including coordination, surveillance, preparedness, and response are taken into consideration. It also includes an IHR Decision Instrument.
Within the Health Portfolio, the Health Portfolio Emergency Preparedness Committee is responsible for overseeing the coordination and joint integration of emergency preparedness activities. The committee also supports the minister of health in meeting obligations under the Emergency Management Act. The key responsibilities of this group are to:
- Identify priorities and provide direction in emergency management across the Health Portfolio, including the development of emergency preparedness policies, plans, and exercises of common interest.
- Provide leadership and strategic oversight to ensure appropriate policies and/or measures are in place, including the capacity and capabilities to mitigate, prepare for, respond to, and recover from emergency situations
Under the Emergency Preparedness Committee is an Operational Subcommittee, which coordinates, approves, and facilitates linkages between operational activities and time-limited projects related to different aspects of preparedness, such as planning, training, exercises, lessons-learned, and risk and capabilities assessments.
Canada's National Microbiology Laboratory has an Emergency Management Program that is defined by the four phases of emergency management-preparedness, mitigation, response and recovery. As part of its Emergency Management Program, the National Microbiology Laboratory maintains a sophisticated operations centre, comprehensive response plans, and a robust training and exercise program for its entire staff. It works closely with its provincial counterparts through the Canadian Public Health Laboratory Network to prepare for and respond to emergent public health events as a cohesive laboratory system (see section D1: National laboratory system).
International plans
Canada's International Emergency Response Framework is maintained by Global Affairs Canada. It was developed to provide an integrated all hazards approach, across the federal government, to prepare for and respond to international emergencies. The purpose of the Framework is to enable prompt, coordinated and effective action, facilitating interoperability and the mobilization of appropriate resources on a timely basis to international emergencies affecting Canadians abroad.
Canada also maintains plans and agreements with other countries to respond to emergencies. For example, Canada is a partner in the trilateral North American Plan for Animal and Pandemic Influenza. It outlines how Canada, Mexico, and the United States intend to strengthen their emergency response capacities and trilateral collaborations and capabilities for a faster, more coordinated response to an outbreak of animal influenza or an influenza pandemic in North America.
Human resources to support emergency response centres
Federal, provincial, and territorial governments all maintain appropriate systems to ensure personnel are trained and available to respond to emergencies when they arise. For example, the federal Health Portfolio maintains a roster of experienced and qualified personnel who can be mobilized from their day-to-day program areas into a Health Portfolio emergency response structure established to respond to a public health emergency (within the Health Portfolio Operations Centre, the National Microbiology Laboratory Operating Centre, or a Regional Emergency Coordination Centre).
Provinces and territories are also able to share health professionals to provide surge capacity during emergencies through mutual aid agreements, including the Operational Framework for Mutual Aid Requests (see section R4: Medical Countermeasures and personnel deployment).
Reviewing, exercising and updating plans
Exercises are conducted each year across Canada at the federal, regional, provincial, territorial, and municipal levels to test the efficacy of existing plans and procedures and adapt them as needed.
At the federal level, the Health Portfolio follows an exercise planning cycle, which is a tool for prioritizing exercise activities and aligning efforts and resources. The planning cycle is informed by:
- Departmental mandates
- Priorities set out by the Government of Canada
- Lessons learned from after-action reviews
- Direction to prepare for upcoming events
- A broad range of stakeholder commitments
The Public Health Agency of Canada's Lessons Learned Unit conducts after-action reviews to inform discussions on capability improvements.
Under the National Microbiology Laboratory's Emergency Management Program is a cyclical review that includes the exercising and updating of its plans, equipment, and personnel. After every event or exercise the Laboratory compiles an after-action review that generates an improvement plan and corrective actions. These are entered into the Laboratory Information Management System as part of the Laboratory's Quality Program, and tracked to ensure that corrective actions are completed.
Within Public Health Agency of Canada's regional offices, after action reviews are conducted following each exercise and event. Recommendations are recorded in after-action reports, lessons learned documents and improvement plans, depending on the scope and scale of the exercise or event. Recommendations are tracked and implemented according to the improvement plans.
Indigenous Services Canada has a Communicable Disease Emergency (CDE) planning cycle and tabletop exercise toolkit for First Nations communities. The Department works with First Nations communities to regularly test and update community Communicable Disease Emergency plans, which are, ideally, integrated with all hazard plans.
Provinces and territories also routinely test emergency management plans through operations and exercises. Many of the recent tests at the provincial and territorial levels have focused on mobilizations of personnel and stockpiles. All jurisdictions have indicated that lessons-learned processes are in place and routinely used to update plans and standard operating procedures as required.
R.1.2 Priority public health risks and resources are mapped and utilized
Public Safety Canada and Defence Research and Development Canada lead the development of a National Risk Profile for the Government of Canada. A Federal Risk Assessment Working Group has been established to work on the National Risk Profile on an ongoing basis. The priorities of the Working Group are set on an annual basis.
The federal Health Portfolio has developed a planning and capability enhancement cycle, which is described in detail in Appendix B of the Health Portfolio Strategic Emergency Management Plan. The purpose of this cycle is to facilitate the process whereby each completion of risk assessment, training, exercises, response to and recovery from public health events, after-action reviews, and lessons learned builds on the last to continually improve the Health Portfolio's emergency planning and readiness.
Stockpiles
Stockpiling of assets for health emergencies is a shared responsibility of federal, provincial and territorial governments, and each level of government has adopted standard operating procedures and policies to meet the needs of their populations based on their unique risk assessments.
The National Emergency Strategic Stockpile is a federally owned reserve of emergency supplies that are intended to provide surge capacity in support of the provincial or territorial response during events. The Stockpile is designed to help respond to all types of hazards-from CBRNE incidents to a broad range of natural disasters. The Stockpile includes a wide spectrum of pharmaceuticals, medical supplies and emergency response equipment (see section R4: Medical countermeasures and personnel deployments).
Stockpile assets have been deployed to support multiple events in recent years. Protocols and procedures have been tried and tested to ensure efficiency. The Stockpile has a series of standard operating procedures for the deployment and management of health emergency assets. Resources include emergency social service assets, such as beds and blankets; health assets, such as mini-clinics to support triage and medical care; and medical countermeasures, such as antitoxins, antibiotics, and vaccines.
Provinces and territories adopt appropriate measures to stockpile emergency health supplies within their respective jurisdictions. Many provinces maintain stockpiles of equipment and supplies to complement assets in the National Emergency Strategic Stockpile. There is an ongoing effort to share information across jurisdictions to ensure an appropriate complement of supplies, avoid duplication, and coordinate the purchase of new or replacement stocks. As well, Indigenous Services Canada maintains a stockpile of personal protective equipment for health care providers in First Nations communities during an emergency.
In the event of an emergency, there are many different types of bilateral, regional, and national mutual aid agreements that can be leveraged to share both supplies and personnel.
Best practices, challenges, gaps and recommendations
Canada continues its preparedness work through nation-wide planning and exercises to strengthen formal and informal communication networks across federal government departments, provincial and territorial governments, and other relevant stakeholders. Canada is geographically vast, with unique hazards, risks and capacity that vary across the country. The successful adoption of the Federal, Provincial and Territorial Public Health Response Plan for Biological Events should be viewed as a best practice to ensure integrated response across jurisdictions during emergencies.
Canada has developed strong and effective systems for deploying assets and personnel across the country, but at times has faced challenges when engaging in similar activities internationally. In 2016, the federal Health Portfolio adopted the Health Portfolio Mobilization Strategy for Event Response, which includes concrete actions to improve policies and processes related to the international deployment of personnel. A policy review is currently underway to streamline these processes.
The federal Health Portfolio has demonstrated that it has the capacity to respond to emergencies with health consequences. However, the training of staff, particularly on emergency management plans and procedures, has been highlighted as a common shortfall during after-action reviews. As part of the implementation of the Health Portfolio Mobilization Strategy for Event Response, a revised training program is being set up to address this area for improvement.
Some of the challenges noted by provincial and territorial jurisdictions related to preparedness include:
- Difficulty mobilizing assets quickly during emergencies due to severe weather conditions and geographic distance and remote location
- Supply chain vulnerabilities for certain pharmaceuticals, particularly if guidelines regarding personal protective equipment and vaccine administration change during an event
- Ensuring timely communication and awareness of federal supports available to provinces and territories during emergencies
It is important to note that when these challenges arise during emergencies, structures and forums are in place to help resolve them and to identify solutions to meet the needs of the event.
R2: Emergency response operations
Joint external evaluation target: Countries will have a public health emergency operations centre functioning according to minimum common standards; maintaining trained, functioning, multi-sectoral rapid response teams and "real-time" biosurveillance laboratory networks and information systems; and trained emergency operations centre staff capable of activating a coordinated emergency response within 120 minutes of the identification of a public health emergency.
Level of capability in Canada
In Canada, first responders at the local level are responsible for initiating responses to public health emergencies. In significant events, where local capacities require additional assistance to respond, requests for support are directed to the provincial or territorial level. Provincial and territorial authorities will lead the response to the health emergency within their jurisdiction, and it is only when they require additional support in coordinating the response, or additional surge capacity that the federal level will be engaged.
Canada has well established networks and procedures in place to facilitate real-time information sharing across jurisdictions, including 24/7 watch offices across different levels of government that are on call to respond to events as they develop. As indicated above, response in Canada generally escalates from local, to provincial/territorial, to federal levels depending on the scope of activities and the severity of the event.
In this context, the 120-minute activation target is not well suited to Canadian operational realities. In almost all cases, the local system will respond immediately to public health events, and as issues are flagged, greater coordination will occur as required. In some cases, activations and emergency operations centre related activities will be coordinated through virtual spaces, meaning they will be addressed through video and teleconferences among relevant stakeholders across the province, region and country.
In general, Canadian emergency management programs do not measure performance against the
120-minute target, but have proven through multiple recent operational responses and exercises that activities can be coordinated and addressed quickly and effectively as events unfold.
Emergency operations centres across Canada at the municipal, provincial and territorial, and federal levels have operating policies and procedures designed to reflect their unique jurisdictional needs and realities. Many emergency operations centre procedures and plans are routinely tested either through operational activities or through exercises (see section R1: Preparedness).
A range of lessons-learned and after-action review processes are in place to ensure procedures are continually updated to meet operational challenges. Furthermore, efforts are made at all levels of government to ensure policies are in place to enable the effective sharing of information at all levels of activations.
Provincial, territorial and federal governments have complementary roles in emergency management, and each jurisdiction has emergency management legislation which details its own responsibilities. There are various programs, structures and collaborative networks in place to ensure all of the legislated duties are met.
Emergency operations programs in Canada are dependent upon strong communication networks across jurisdictions and the maintenance of 24/7 watch office or surveillance capacity to ensure timely interventions when needed.
Each province and territory has standard operating procedures related to the transportation and management of infectious patients. These procedures follow principles of good patient care and utilize "routine practices" provided by the Government of Canada. The transport of patients at the local level is the responsibility of the province or territory and is mainly done through paramedic services.
The federal government is responsible for health screening at points of entry and has programs in place for reporting and referring cases to local public health authorities. Special procedures are also in place for the transportation and management of highly pathogenic diseases.
Finally, there are a number of mutual-aid agreements in place across Canada at the regional, national, and international levels that facilitate the sharing of personnel and assets to assist local health authorities during emergencies and ensure that cases are appropriately managed.
Indicators
R.2.1 Capacity to activate emergency operations
Triggers and activation levels for all hazard emergency operations centre activation
At the provincial and territorial level, an emergency response is activated immediately at the local level with on-site emergency responders. The triggers for a municipal, provincial or territorial emergency operations centre response will be based on the jurisdiction's plans and procedures. Hospitals, regional health authorities, ambulance services, and other entities in the provincial and territorial health sector also maintain emergency response operations and plans.
At the federal level, public health response to an event is rarely triggered by an immediate crisis. More often, the federal government's role in supporting emergencies is one that evolves gradually over time. Provinces report on the status of emerging events and relevant federal departments will respond following procedures. Once activated, however, the Health Portfolio Operations Centre will coordinate federal response activities, allocating resources as the needs of the event emerge.
When a certain threshold is reached, the following are considered: triggers for activation, the objectives of the response, and the appropriate level of senior management engagement. The recommendation for activation is made in accordance with the Health Portfolio Emergency Response Plan. Once a decision is taken to activate the incident management structure, the Health Portfolio Operations Centre can quickly pull together a response team.
At the pan-Canadian level, the Federal, Provincial and Territorial Public Health Response Plan for Biological Events details a concept of operations that describes the steps taken from the initial notification of a public health event leading to the activation of the plan to the eventual de-escalation of the response. It describes how notification of a public health event is made to PHAC and how response needs are assessed by federal, provincial, and territorial technical experts and decision-makers.
The concept of operations also describes four response levels to facilitate scaling of activities as needed. It details activation triggers that are assessed to determine whether enhanced federal/provincial/territorial coordination is necessary. The capacity of jurisdictions to participate in the federal/provincial/territorial governance structure, and engage through activation of the plan, is determined by each respective jurisdiction. Provinces and territories may have limited ability to participate due to resource demands in their own jurisdictions.
Emergency operations centre personnel
Provinces and territories maintain plans to recruit and train emergency operations centre staff as required. Some jurisdictions have stand-alone health operation centres, while others are integrated within the broader jurisdictional emergency management system.
Provinces and territories provide comprehensive training for emergency operations centre staff and personnel as required to maintain operational readiness and according to its respective emergency response structure (e.g. incident management system or incident command system).
At the federal level, Public Safety's Government Operations Centre supplements its staffing during an event with liaison officers provided by other government departments who are embedded in the Government Operations Centre's event team.
When the Health Portfolio Operations Centre is activated to respond to an event, the incident management structure is staffed by Health Portfolio employees who are temporarily assigned to provide short-term surge capacity. During after-action reviews, employees highlighted that at times they were asked to support the incident management structure with little emergency management training ahead of time.
To address this gap, as part of the Health Portfolio Mobilization Strategy for Event Response, a new training program has been developed for all staff interested in being mobilized to support incident management structure and will be implemented in 2018.
The National Microbiology Laboratory Operations Center uses an incident command system and is staffed 24/7 by 12 trained operations centre directors on a rotational basis. The National Microbiology Laboratory trains its entire staff in the incident command system and emergency management in order to ensure a sufficient number of personnel are available to staff the emergency operations centre.
Regional Emergency Coordination Centres are located in PHAC regional offices. Staff roles are defined in the Regional Health Portfolio Emergency Response Plans in accordance with the principles of the incident command system and the incident management structure functions.
Once activated, Regional Emergency Coordination Centres may be capable of supporting 24/7 operations, depending on the nature of the emergency or event, but require additional human resources from other regions to maintain operational requirements for prolonged periods. Each region can determine how to deliver emergency operations centre training to its personnel, providing the learning outcomes conform to national guidance.
Response teams have been trained using a combination of on-line, classroom, and practical experience delivered by in-house means, external contractors or other government departments and multi-national partners (e.g., United States Centers for Disease Control and Prevention, United Nations).
R.2.2 Emergency operations center operating procedures and plans
Emergency operations procedures
All municipalities, provinces, and territories maintain plans and operational manuals that support emergency operations centre staff in performing their functions. Operating procedures vary by jurisdiction and legislation and reflect the emergency response system used by that jurisdiction. At a pan-Canadian level, the Federal, Provincial, and Territorial Public Health Response Plan for Biological Events illustrates the operational communications that will take place between the Health Portfolio Operations Centre and provincial and territorial emergency operations centres during a national emergency.
At the federal level, the Health Portfolio maintains a suite of response plans and operating procedures. The Health Portfolio Emergency Response Plan outlines operational and planning guidelines to enable the Health Portfolio to coordinate the delivery of support capabilities to provincial, territorial, other federal departments, and international partners during an emergency. The Plan is a key element in the Portfolio's overall emergency preparedness and response program. It is a generic "all hazards" plan that defines the framework within which the Health Portfolio will operate to ensure an appropriate response to any emergency.
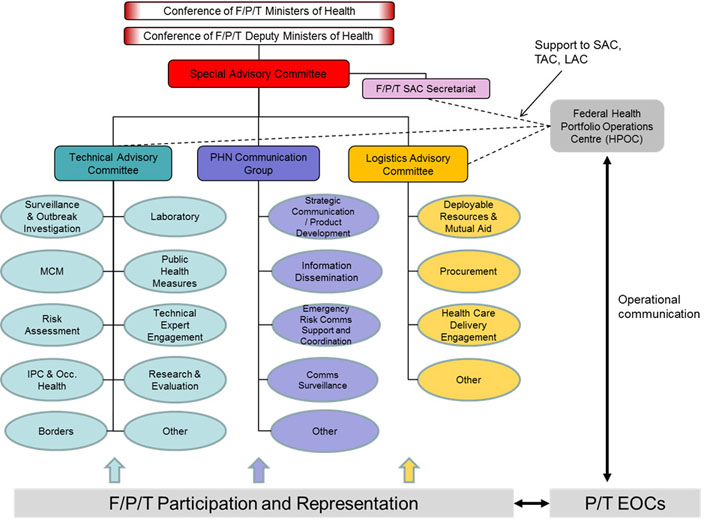
Figure 6: Governance structure for a coordinated response to a biological threat in Canada - Text description
Figure 6 outlines the governance structure for a coordinated response to a biological threat in Canada and illustrates the operational communications that will take place between the Health Portfolio Operations Centre and provincial and territorial emergency operations centres during a national emergency.
At the top of the chart is the Conference of F/P/T Ministers of Health. Reporting directly to this group is the Conference of F/P/T Deputy Ministers of Health. Falling underneath the Deputy Minsters is a Special Advisory Committee (SAC), represented by a red box. The SAC is supported by the F/P/T SAC Secretariat, depicted by a purple box.
Stemming from the SAC are 3 main bodies:
- The Technical Advisory Committee (TAC)
- the PHN Communications Group
- and the Logistics Advisory Committee (LAC)
The TAC (represented by a teal box) provides expertise and support in a range of areas, represented by teal ovals. These include:
- Surveillance and Outbreak Investigation
- Laboratory
- MCM
- Public Health Measures
- Risk Assessment
- Technical Expert Engagement
- IPC and Occupational Health
- Research and Evaluation
The PHN Communications Group (represented by a blue box), provides an array of services and advice, represented by blue ovals. These include:
- Strategic Communication/Product Development
- Information Dissemination
- Emergency Risk Comms Support and Coordination
- Comms Surveillanc.
The last group, the LAC (represented by a yellow box) covers a variety of areas, represented by yellow ovals. Listed are:
- Deployable Resources and Mutual Aid
- Procurement
- Health Care Delivery Engagement
- Other
At the bottom of the figure is a long box depicting F/P/T Participation and Representation, with arrows pointing upwards toward the 3 streams just described, indicating that these groups are made up of multi-jurisdictional delegates.
There is a double sided arrow leading from the F/P/T Participation and Representation box to a box on the right entitled "P/T EOCs" to highlight that there is flow of information between these entities.
A double sided arrow leads from the P/T EOCs to a box in the top right corner of the page representing the Federal Health Portfolio Operations Centre (HPOC) to illustrate the operational communications that will take place between the HPOC and provincial and territorial emergency operations centres during a national emergency.
Lastly, there are dotted lines leading from the HPOC back to the Technical Advisory Committee, Logistics Advisory Committee, and the F/P/T Special Advisory Committee Secretariat, to demonstrate that the HPOC provides support to these bodies during a national emergency as well.
The foundation of the Emergency Response Program is a seven-phase response process with guidance tools that are designed to support a well-coordinated Health Portfolio emergency response. The process is presented in a linear fashion, recognizing that during an emergency, the process may need to be shortened or modified depending on the nature and severity of the threat or hazard.
Operational procedures and protocols in place are reviewed annually. For example, the Health Portfolio Operations Centre's Concept of Operations, Standard Operating Procedures, and Job Action Sheets provide the detailed operational guidelines for a Health Portfolio response to events and emergencies, following the establishment of the Health Portfolio Emergency Response Structure.
Similarly, at the regional level, the Regional Health Portfolio Emergency Response Plan and the Regional Emergency Coordination Centre's Concept of Operations, Standard Operating Procedures and Job Action Sheets are also in place to provide the operational guidelines and detailed roles and responsibilities.
The National Microbiology Laboratory has a set of standard operating procedures. These include positional work instructions for each incident command system position, emergency response plans, and memoranda of understanding. To maintain its ISO 9001 accreditation, the National Microbiology Laboratory submits its standard operating procedures to a rigorous update process at least once every year. Records of procedures are maintained and distributed through the National Microbiology Laboratory's Laboratory Information Management System and Quality Programs.
Frequency of updates and records maintenance and distribution
Emergency response plans at all levels in Canada are continually edited and updated based on after-action reviews of responses-to real or simulated events-that required the implementation of plans.
For example, the Federal, Provincial and Territorial Public Health Response Plan for Biological Events will be reviewed at least every three years by the Public Health Infrastructure Steering Committee of the Public Health Network Council. All changes will be tracked and noted as amendments in the plan. A time-limited Infrastructure Steering Committee task group may also be established to conduct this work, which would include recommendations for the development of new event-specific annexes, as required, to further support implementation of the plan.
At the federal level, the Federal Emergency Response Plan is also formally reviewed based on lessons learned through exercises and actual events, and is intended to be republished annually. The review includes assessing the relevance of existing event-specific plans, developing new event-specific plans as required, and maintaining operational processes consistent with changes to federal departmental roles and responsibilities.
At the federal level, the Health Portfolio Emergency Response Plan is reviewed and amended on a regular basis to ensure that it reflects any changes in legislation, policy, or priorities and to address lessons learned as a result of implementation. Records of procedures are maintained on a shared drive and are also distributed through e-mail when an employee is approved to work in the incident management structure.
The Regional Health Portfolio Emergency Response Plan and its associated procedures are updated on a regular basis in alignment with the national Plan. Proposed changes are reviewed annually, or as after-action reviews or legislation changes warrant. Maintenance, review, and revision of the Regional Plan and its associated procedures is led by the Regional Coordinator and approved by the Regional Emergency Preparedness Committee or Health Portfolio Emergency Preparedness Committee as required. Changes are tracked and noted as amendments and disseminated to the Regional Emergency Preparedness and Response Units.
R.2.3 Emergency operations program
In Canada, emergency operations programs are in place at each level of government, and in many cases, procedures are built in and regularly tested through routine operations (for example, in hospitals). Federal and regional capabilities are based upon national program guidance and direction.
When activated, response organisations work with federal, provincial and territorial emergency management authorities to ensure whole-of-government response coordination and collaboration. This guidance is detailed in the National Emergency Response Framework. An overview of some of the key emergency operations centres and programs that exist across Canada are provided under the different jurisdictional levels identified below.
Federal emergency operation programs
Public Safety Canada houses the Government Operations Centre, which provides an all hazards integrated federal emergency response to events (potential or actual, natural or human-induced, accidental or intentional) of national interest. It provides 24/7 monitoring and reporting, national-level situational awareness, warning products and integrated risk assessments, as well as national-level planning and whole-of-government response management. This includes responses to public health, essential human services, and agriculture or agri-food emergencies in collaboration with Health Canada, PHAC, and the Canadian Food Inspection Agency respectively.
The Public Health Agency of Canada manages the Health Portfolio Operations Centre, which maintains a 24/7 state of readiness and serves as the Health Portfolio "single window" for the coordination of all hazard response activities. The Operations Centre expedites and facilitates information sharing with key stakeholders, and communicating and coordinating with other operation centres across federal and provincial and territorial levels of government. The Operations Centre also works with the Health Portfolio Office of International Affairs, located within PHAC, to collaborate and communicate with international stakeholders to support responses to international emergencies with health consequences.
To maintain strong working relationships with provincial and territorial partners, and recognizing the diversity of contexts that exist across the country, PHAC also has Regional Emergency Coordination Centres in six regions across Canada. The Regional Centres act as liaisons with provincial and territorial partners, facilitate the communication of provincial needs, and coordinate the delivery of federal support, taking into account the unique circumstances of each region.
Projects are underway to upgrade these centres, and regions have had positive feedback on much of the work that has been implemented so far. However, budget constraints have negatively impacted operations in some regions, particularly those regions that cover multiple provincial and/or territorial jurisdictions that are themselves facing resource pressures.
The National Microbiology Laboratory's Operations Centre acts as the Laboratory's 24/7 single window for emergent events. It coordinates laboratory activities related to infectious disease emergencies in collaboration with the Health Portfolio Operations Centre. The National Microbiology Laboratory Operations Centre monitors sources of public health intelligence to provide early warning of an event that may pose a public health risk and provides the materiel and human resources to manage the laboratory response within the incident command system.
This support is provided through the National Microbiology Laboratory's laboratory programs; the Canadian Public Health Laboratory Network's mobile laboratory response teams: the Microbiological Emergency Response Team and the Special Pathogens Diagnostics Response Team, which responds both domestically and internationally to disease outbreaks or biological agent threats (see section D1: National laboratory system).
The Health Portfolio Operations Centre, the National Microbiology Laboratory Operations Centre and the six Regional Emergency Coordination Centres use incident management systems to ensure effective, efficient, and collaborative incident management. The incident management system is based on the standardized concept of the incident command system, which is an integrated organizational structure that can be easily adapted to the requirements of an event. It facilitates coordination between the Health Portfolio and other governments and agencies, including the Government Operations Centre, while also adapting to the needs of each organization.
Provincial and territorial emergency operations and programs
Provinces and territories all have emergency response programs in place which are linked in different ways to their health systems. Provincial and territorial health systems have embedded process, surveillance, and reporting channels to share information on emerging events. Many provinces have also adopted response systems based on an incident management system or incident command system.
As an example, Alberta uses an incident management system that comprises a series of escalating responsibilities from the "bottom up." The intention is to resolve the emergency at the lowest possible level until it has been determined that the community can no longer manage the event, or requires assistance.
The full spectrum of emergency management in the province begins with individual responsibilities for safety and security and escalates through assigned roles and responsibilities of formally organized emergency management partners. These include, but are not limited to, first responders, communities and their mutual aid partners, regional associations, provincial government, and federal and international assistance in the most serious and widespread disasters. Industry and non-governmental organizations are key partners in the system, supporting emergency management activities at almost every level.
Another example is New Brunswick Health, which has an emergency operations centre that acts as a strategic headquarters responsible for planning, directing, aligning and coordinating emergency response recovery actions to health emergencies. For impacts that affect the entire provincial health system, the authority for the overall management of emergency events, including control of the health system's resources will be exercised by the New Brunswick Health Operations Centre.
The response to an emergency that poses a direct threat to the health system or puts increased demands on the system, such as a mass casualty event or the loss of a facility, will normally take a "ground up approach." This refers to having the emergency handled at the lowest level possible with support provided, as required, from regional health system partners, the Department of Health, the provincial and/or the federal government.
The Department of Health is also able to activate all or part of the health system in response to slowly emerging wide-spread threats, such as communicable diseases, or in response to requests for resources from the federal government, other provinces, or neighbouring States.
R.2.4 Case management procedures are implemented for IHR relevant hazards
Management and transportation of infectious patients
The management and transportation of potentially infectious patients is the responsibility of the provinces and territories and designated to local paramedic services. Each province and territory has standard operating procedures related to the transportation and management of infectious patients. These procedures follow principles of good patient care and use "routine practices" provided by PHAC.
The Health Portfolio Operations Centre Watch Office also has protocols in place for the transport of potentially infectious patients at the local level and point of entry. PHAC's Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Healthcare Settings provides guidelines to prevent the transmission of microorganisms to patients, healthcare workers and visitors. It is intended to assist healthcare organizations, infection prevention and control professionals, and all other healthcare providers responsible for developing policies and procedures related to routine practices in all settings where health care is provided. This includes acute or long-term care, ambulatory care, home care or prehospital care settings.
For enhancing the Health Portfolio's ability to respond to chemical events, the Chemical Emergency Preparedness and Response Unit located within Health Canada has developed an IHR guidance tool for the risk characterization and reporting of chemical events. (See section CE: Chemical events,)
For highly pathogenic diseases, Canada has recognized the need for special transport measures and collaboration across levels of government. For example, the Government of Canada developed the Transport Canada Civil Aviation Contingency Plan for Pandemics and Communicable Disease Events and other standard operating procedures to deal with the transport of infectious patients during an Ebola outbreak. The general intent of this Plan is to ensure that the Civil Aviation response to a pandemic or communicable disease event is appropriate and adequately addresses the declared phase and subsequent issues.
Special measures are also in place to screen people arriving at points of entry in Canada. At designated points of entry, a quarantine officer can refer patients after conducting a health assessment either person or remotely with the assistance of a screening officer. For additional information, see POE: Points of entry.
Best practices, challenges, gaps and recommendations
The Health Portfolio Operations Centre has been activated regularly in recent years, at times dealing with simultaneous events. Canada has demonstrated that it has the capacity to effectively respond to events with health consequences both within Canada and internationally.
Having recognized gaps in some of its current practices, the federal Health Portfolio is in the process of implementing new recruitment and training programs, ensuring there is a broad range of trained human resources available to respond to events when they arise.
At the regional level, PHAC Emergency Preparedness and Response Units have noted that recent upgrades to Regional Emergency Coordination Centres have enhanced their operational capacity, and the project underway to upgrade all of these Centres is seen as a positive development to enhance coordination and engagement in collaborative activities.
Challenges in northern, remote and isolated communities related to infrastructure (telecommunications, transportation, power) and human resources continue to have an impact on service delivery, including emergency management and some inter-jurisdictional coordination and collaboration.
R3: Linking public health and security authorities
Joint external evaluation target: In the event of a biological event of suspected or confirmed deliberate origin, a country will be able to conduct a rapid, multi-sectoral response that includes the capacity to link public health and law enforcement; provide and/or request effective international assistance; and investigate alleged use events.
Level of capability in Canada
Canada has established strong linkages between public health and security and intelligence authorities to provide Canadians with a comprehensive and coordinated approach to planning for and responding to public health emergencies, including bioterrorism. These linkages are supported by mechanisms that incorporate and coordinate the three overlapping areas of a terrorism response: crisis, security and intelligence, and consequence management.
As an example, the Security of Canada Information Sharing Act provides a means for the Canadian security and intelligence community, the Public Health Agency of Canada, Health Canada and the Canadian Food Inspection Agency, to share information on threats to national security, including public health-related threats.
Further, a number of formal memoranda of understanding and other agreements between national public health authorities and members of the security and intelligence community help define roles and responsibilities during an emergency that may affect public health. Some of these may further define information-sharing roles.
There are also memoranda of understanding between federal and provincial/territorial public health authorities that address information-sharing during a public health emergency. At the provincial and territorial level, most public health jurisdictions maintain either memoranda of understanding, agreements, protocols, or plans for engaging with and sharing information with law enforcement for specific public health emergencies such as bioterrorism.
A series of plans for responding to public-health emergencies, including intentional chemical, biological, radiological, nuclear and explosives (CBRNE) events are in place across Canada. These plans to mitigate bioterrorism risks are based on information from comprehensive, all hazard risk assessments. Plans are exercised and validated on a regular basis, often with participation from federal, provincial and territorial public health authorities, and non-governmental and international partners.
Indicators
R.3.1 Public health and security authorities, (e.g. law enforcement, border control, customs) are linked during a suspected or confirmed biological event
Legislative and policy infrastructure and plans
Canada has in place many agreements at the national level to ensure that public health and security partners are coordinated throughout all phases of a terrorist event. The Building Resilience Against Terrorism: Canada's Counter-terrorism Strategy represents Canada's commitment to the principal that a terrorism response requires an integrated approach by the Government of Canada, all levels of government, law enforcement agencies, the private sector, and citizens, and collaboration with international partners and key allies, such as the United States.
Supporting this overarching strategy, all levels of government have collaborated to develop the Chemical, Biological, Radiological, Nuclear and Explosives Resilience Strategy for Canada. Its purpose is to provide the policy framework that will guide the creation of sustainable capabilities and common standards in CBRNE policies, programs, equipment and training. Both strategies are aligned with the Emergency Management Framework for Canada described in section R1: Preparedness.
In addition to these strategies, Canada has in place plans that address response to possible biological events and other public health events that may have deliberate motives.
At the national level, a Federal Terrorism Response Plan has been developed to strengthen coordination among security and intelligence agencies and all levels of federal government by bringing them together under one coherent plan for responding to terrorism. It is designed to address domestic terrorist incidents, and acts of terrorism committed abroad that implicate domestic security and intelligence agencies. Its focus is the security and intelligence response and its links to crisis response and consequence management coordinated under the Federal Emergency Response Plan (FERP).
The FERP is an all hazards response plan for use by the Government of Canada to coordinate consequence management for acts of terrorism including bioterrorism. The FERP sets out the mechanism for federal support in a terrorist event. The Public Safety Canada's Government Operations Centre plays a lead role in coordinating the federal response and providing situational awareness across departments. This includes coordination of information flow between security and intelligence and emergency management partners. Provinces and territories are linked to the Government Operations Centre through their respective emergency management organizations.
Annexed to and supporting the FERP are 13 emergency support functions. These provide a mechanism for ensuring that public health and security authorities are linked in a whole of government response to a biological event at a national level.
Emergency support functions provide a mechanism for grouping the functions most frequently needed to respond to requests for assistance during an emergency and are allocated to primary federal government institutions consistent with their mandates and areas of responsibility.
For example, ESF# 13 Border Services (led by Canada Border Services Agency) and ESF# 8 Law Enforcement (led by the Royal Canadian Mounted Police), support and in turn are supported by ESF#5 Public Health and Essential Human Services, (led by the federal Health Portfolio). All three emergency support functions may be implemented by the Government of Canada in the enforcement of the Quarantine Act in a bioterrorism event. (See section POE: Points of entry.)
The FERP is further supported by federal departmental emergency response plans, including the Health Portfolio's all hazard Health Portfolio Emergency Response Plan. (See section R1: Preparedness.)
The Health Portfolio Emergency Response Plan would be used by the federal Health Portfolio to coordinate the federal public health response to an act of bioterrorism. If federal support was requested by a province or territory, the Health Portfolio would also implement the Health Portfolio CBRNE Intentional Events Annex, which is the hazard-specific annex to the Plan.
The Health Portfolio Emergency Response Plan describes how, if the provincial/territorial health sector requires support for response to an intentional biological event, the Health Portfolio Operations Centre will coordinate the provision of federal scientific and public health support to assist the province/territory in its consequence management response efforts.
The Operations Centre will also manage classified information with its partners, as required, in accordance with its standard operating procedures. The Annex provides for linkages to security partners through the sharing of liaison officers between the Health Portfolio Operations Centre and the Royal Canadian Mounted Police's National Operations Centre. (See section R2: Emergency response operations for more details.)
To support national collaboration between all levels of government, the health sector has in place the Federal, Provincial, and Territorial Public Health Response Plan for Biological Events. The Plan facilitates formal coordination of responses to public health events that are biological in nature and of a severity, scope or significance to require involvement of senior level decision-makers at a national level.
While linkages to the law enforcement and security are outside the scope of the Plan, it is expected that the national governance structure for a biological event where the intent is malicious, would be similar to that as described in the Plan.
Global Affairs Canada developed Canada's International Emergency Response Framework to guide the country's response to terrorist-related critical incidents abroad that involve Canadians and Canadian interests and to facilitate collaboration with international partners. The Plan describes how Global Affairs Canada leads all official efforts related to coordinating the international dimensions of a terrorist incident in Canada, with the support of other federal departments. Global Affairs Canada is also the lead department responsible for managing requests for, and offers of, international assistance in a terrorism event.
In addition to these plans, Canada has in place several memoranda of understanding and other agreements between public health and security authorities at the federal level. For example:
- Memorandum of Understanding between the Public Health Agency of Canada and the RCMP concerning the operations of the national CBRNE response team in response to the criminal or terrorist use of biological materials
- Memorandum of Understanding between the Public Health Agency of Canada and Department of National Defence concerning the storage of smallpox vaccine and vaccinia immune globulin
- Memorandum of Understanding between RCMP and the Public Health Agency of Canada for provision of air transportation services by the RCMP to the Public Health Agency of Canada in the event of a national emergency
- Memorandum of Understanding between the Public Health Agency of Canada, Health Canada and Transport Canada Civil Aviation on the information exchange procedures dealing with any person, cargo or other thing on board an aircraft that could cause the spreading of a communicable disease
- Umbrella Memorandum of Understanding between the Canada Border Services Agency and Health Canada to outline our understanding of cooperation with respect to the administration and enforcement of acts and regulations relating to travelers, conveyances, cargo and certain controlled, prohibited, or regulated goods
Information sharing and training
At the federal level, government departments must share information efficiently across all levels and sectors to effectively detect terrorist threats. This means communicating with provinces, territories and municipalities; Canada's allies; non-traditional and international partners; and private sector stakeholders.
Public Safety Canada and the Department of Justice lead the development of legislative proposals to improve information-sharing among departments and agencies for national security purposes. Proposals must be consistent with Canada's Charter of Rights and Freedoms and the Privacy Act.
In Canada, the Security of Canada Information Sharing Act provides a legislated mechanism at the national level for the sharing of information among members of the Canadian security and intelligence community, including the Public Health Agency of Canada. The purpose of the act is to encourage and facilitate the sharing of information among Government of Canada institutions to protect Canada against activities that undermine national security.
The Public Health Agency of Canada has a memorandum of understanding with one specific member of the Canadian security and intelligence community, which provides the Agency with access to classified information from several core federal security authorities in Canada. In addition, the Agency has well-established working relationships with several departments in the Canadian security intelligence community, including Public Safety Canada, Canada Border Services Agency, the Royal Canadian Mounted Police, the Canadian Security Intelligence Service, and the Canadian Food Inspection Agency for the purposes of information sharing and collaboration.
This access assists the Agency with threat and risk assessments and exercise planning. In addition, the Public Health Agency of Canada's National Microbiology Laboratory maintains a memorandum of understanding with the Royal Canadian Mounted Police regarding CBRNE response support referred to in the previous section.
Within the federal government, there are many other memoranda of understanding between public health and other sectors that promote information sharing and collaboration. For example:
- Memorandum of Understanding between the Public Health Agency of Canada, Health Canada and the Canadian Food Inspection Agency for Common Issues Related to Human Health
- Memorandum of Understanding between the Public Health Agency of Canada and the Canadian Food Inspection Agency Concerning Food Safety on Passenger Conveyances and Ancillary Services
The Department of National Defense Health Services provides representation to the Public Health Agency of Canada's Health Emergency Management Consultative Group to maintain lines of communication.
Not specific to sharing of public health Information, the Treasury Board of Canada Secretariat has a draft document entitled Security Event Information Sharing Protocol. This protocol aims to strengthen and streamline information sharing among federal departments in situations of a domestic security threat, event or incident that may require the Government of Canada security community to support whole of government coordination. The protocol seeks to provide guidance and information in the areas of:
- Departmental incident reporting procedures
- Escalation
- Notification methods
- Communication flows
Classified informational and analytical reports are shared between the federal security and the Public Health Agency of Canada daily.
During a public health emergency, security authorities receive situation reports regularly. As well, the Public Health Agency of Canada shares risk assessments on a case-by-case basis with individual security authorities according to their mandate. The Agency is currently developing an intelligence and analytical capacity to enhance strategic risk assessment reporting for broader sharing with the security authorities. Incidents involving security concerns (e.g. dual use) for further enforcement action would be shared with the security authorities. There is regular information sharing between the Public Health Agency of Canada's Centre for Biosecurity and security authorities in Canada.
The Public Health Agency of Canada also receives threat briefings from security authorities. The Agency receives a broad range of classified information concerning domestic and international threats. This classified threat reporting covers tactical, operational, and strategic issues of concern to Canada's national security.
Canada's Integrated Terrorism Assessment Centre analyzes security intelligence from its various partner institutions and produces assessments related to terrorism threats. These threat assessments are then distributed to members of the Canadian security intelligence community, federal partners (including the Public Health Agency of Canada), provincial emergency authorities, first responders, and the private sector.
Integrated Terrorism Assessment Centre assessments are also used in the development of international travel advisories and in the development of threat and risk assessments for Canadian missions, interests, and persons at home and abroad.
Another information sharing agreement among federal, provincial and territorial public health authorities in Canada is the Federal/Provincial/Territorial Memorandum of Understanding on the Sharing of Information During a Public Health Emergency, which establishes a framework for the sharing of information among federal, provincial and territorial governments during a public health emergency.
The agreement binds each party, upon declaration of a public health emergency, to share with other parties timely, accurate and sufficiently detailed information regarding the emergency, including where necessary, case definitions, case information, laboratory results, source and type of risk, number of cases and deaths, conditions affecting the spread of the disease and the health measures employed. This information sharing is subject to the privacy laws applicable to each jurisdiction.
The Multi-lateral Information Sharing Agreement is a federal/provincial/territorial agreement for the exchange of information on surveillance of infectious diseases (which includes routine surveillance, and case management and response activities), and the management of pan-Canadian and multi-jurisdictional public health events, and public health emergencies of international concern.
The arrangements provincial and territorial governments have in place for information sharing among public health and security authorities and collaborating during a public health security event are not uniform. Some are not formalized, while others are very specific plans with ad hoc committees, protocols and agreements in place. One such example is the Alberta Counter Terrorism Plan, which provides direction for counter-terrorism crisis management, and communication linkages among intelligence organizations, provincial ministries and the private sector as required.
The province of Manitoba has an Avian Influenza Coordination Plan that brings together public health, health emergency management, law enforcement and animal health authorities to collectively respond to an avian influenza outbreak in the province.
Prince Edward Island has developed an all hazards plan, which serves as a response framework agreement for department emergency service officers from public health, fire, security, and others for activation of the Joint Emergency Operations Centre.
The province of Ontario has agreements between the Ontario Public Health Laboratories and some law enforcement agencies (including the RCMP) for testing relating to potential biological events.
Some jurisdictions have developed protocols for certain types of hazard and emergencies. For example, in New Brunswick, a protocol for biological threats (i.e. anthrax) sets out the roles, responsibilities, concept of operations and actions in the case of a suspicious package of a biological nature.
Other jurisdictions manage by working closely and as needed with civil defense, RCMP, or provincial and local policing services. In Saskatchewan for example, local policing is automatically called for all CBRNE events to ensure continuity of evidence and custody of samples, and to assist with local controls.
The Quarantine Act applies in the event of a suspected biological public health threat originating from outside of Canada. To address health related issues on ship, in ports and at international borders, local public health connects with staff working in the federally administered Travelling Public Program and vice versa (see section POE: Points of entry).
Provinces and territories also use existing emergency management coordination channels to share information on various subjects if required (i.e. provincial emergency operations centres). For special events in which there are security concerns (e.g. political summits) special agreements may be put in place specific to the event. In Ontario for example, some local police may also have agreements with local public health for protocols during white powder incidents.
Federal quarantine officers have protocols to inform local public health units in the event of ill passengers, and border services agents are used by quarantine services as screening officers. Therefore, a link exists through that route.
The Ontario Provincial Police is a provincially deployed police service that has both contract and non-contract policing roles/responsibilities in municipalities, towns, cities, unorganized townships, etc. that may or may not have a police services board to provide strategic oversight. Some of these boards may have been instructed to or developed arrangements with local health authorities, but that information is not tracked centrally.
The National Microbiology Laboratory regularly conducts joint training exercises with the RCMP to provide biological science assistance during the investigation and response to a deliberate biological event. Beyond that, there is currently no national joint investigations curriculum in place to train public health and law enforcement entities on joint investigations.
During a joint investigation, any information sharing would be done under the legislated authorities to collect and share information. Work towards this began when the Public Health Agency of Canada led a workshop in March 2015 at the Toronto Police College. Attendees were Province of Ontario federal public health and law enforcement stakeholders whose responsibilities included identification, assessment and response to public health events of potential bioterrorist concern.
Several Agency employees have attended the U.S. Centers for Disease Control and Prevention and the Federal Bureau of Investigation training offered in the United States with a view to promoting a similar curriculum in Canada.
Incident identification and risk assessments
Federal Ministers are responsible for developing, testing, and maintaining mandate-specific emergency management plans and identifying risks that are within or related to their area of responsibility, as required under the Emergency Management Act. There is also a requirement to embed a risk-based emergency management cycle within each institution's broader integrated planning processes.
Every municipality, province and territory also conducts risk assessments in accordance with their respective threat/risk environments and legislative frameworks and works with other sectors and levels of government to mitigate those risks.
Ministers of federal institutions are also responsible for monitoring adherence to and implementation of the Federal Policy for Emergency Management. Under the Policy, federal institutions are required to develop strategic emergency management plans related to the institution's areas of responsibility and based on an all hazards risk assessment.
As required by the Policy, the Health Portfolio has in place a Health Portfolio Strategic Emergency Management Plan which outlines the results of the Health Portfolio's public health risk assessment process and includes regional and intentional risks such as terrorism/bioterrorism. (For more information on the Federal Policy for Emergency Management see section R1: Preparedness.)
The Public Health Agency of Canada helps to support mitigation of potential deliberate or naturally occurring biological threats identified through its risk assessment. This is achieved through the mandatory reporting component of the Human Pathogens and Toxins Act and the Human Pathogens and Toxins Regulations and further supported by section 4 of the Canadian Biosafety Standards.
The Public Health Agency of Canada has also sponsored provincial and territorial public health authorities to obtain federal government security clearances that allow them to access classified threat and risk assessments to aid in the exchange of information concerning medical countermeasures.
Federal government departments work collaboratively to share information and conduct coordinated risk assessments on matters related to health security. For example, federal departments with a security and/or intelligence mandate convene Threat Assessment Groups which, when expanded to address consequence management issues, normally include the Health Portfolio, represented by the Public Health Agency of Canada
An agreement on shared risk assessments between the Public Health Agency of Canada and the Canadian Food Inspection Agency is currently undergoing updates.
The identification of deliberate threats to national security (terrorism) is led by individual members of Canada's security intelligence community according their respective mandates. The Deputy Minister Committee for National Security, which is chaired by the National Security Advisor to the Prime Minister, considers a wide range of current and emerging issues affecting Canada's national security.
The Committee facilitates information sharing across the security and intelligence community and coordinates policy and operational responses by government departments and agencies with shared responsibility for national security.
The Minister of Health sits on the Cabinet Committee on Intelligence and Emergency Management where they receive classified and top-secret briefings from the federal intelligence community on emerging threats. Their Committee's mandate is to consider intelligence reports and priorities and to coordinate and manage responses to public emergencies and national security incidents.
The Directors General Emergency Response Committee is led by the Government Operations Centre under the FERP. It brings together senior management officials of federal government departments monthly to discuss emergency management issues, including risks related to deliberate events. This committee provides an information sharing forum at the Director-general level at the preparedness phase and transitions to an emergency response committee in an emergency.
For the Canadian health sector, the concept of operations described in the Federal, Provincial and Territorial Public Health Response Plan for Biological Events serves as a tool for determining the risks posed by biological events. It describes how to notify the Public Health Agency of Canada of a public health event and how risk and response needs are assessed jointly by federal, provincial and territorial technical experts and decision-makers. (See sections R1: Preparedness and R2: Emergency response operations for more detail.)
Examples of exercises
Exercises are part of the ongoing mandate of all federal, provincial, territorial government partners involved in public health emergency preparedness and response. The Public Health Agency of Canada regularly develops exercises to test preparedness and response plans at the national level. Exercises vary in scope and purpose, but cover a wide range of public health emergency scenarios. They involve partners from all sectors that would be required in an actual response, such as federal, provincial and territorial governments, the health care sector, the private sector, and non-governmental organizations.
At the national level, select Public Health Agency of Canada personnel participate in training provided by the RCMP about principles of law enforcement. In March 2017 the Agency hosted a tabletop exercise with specific members of Canada's security and intelligence community to examine information sharing during an event. As well, in April 2017, the Agency participated in a national-level exercise which brought together several members of Canada's security and intelligence community to examine information sharing and response during a major radiological event.
At the national level, Canada is currently in the midst of a series of exercises that are conducted under the National Security themed exercise program to participate in federal consequence management planning. This touches on public health and security (intelligence) authority, acquisition/deployment of medical countermeasures, and health personnel. These exercises have involved senior management and political leadership from both the public health and security authorities. For example, biosecurity and biosafety subject matter experts from the Public Health Agency of Canada's Centre for Biosecurity participated in an anthrax simulation exercise in March 2017.
Exercise STAUNCH MAPLE 2017 was a joint U.S. and Canada military-led exercise that incorporated the consequence management of events leading up to, and following, the detection and detonation of nuclear improvised explosive devices in Canada and the United States.
The exercises ranged from tabletop exercises to command post exercises connecting federal and provincial health and security organizations, operations centres, and senior leadership. Corrective actions identified opportunities to increase the security culture within health organizations, strengthen communications between both sectors at all levels, and increase health input to risk assessment and contingency planning. International exercises included health security exercises associated with the G20 health ministers.
Plans are underway to test the newly approved Federal, Provincial and Territorial Public Health Response Plan for Biological Events in 2018-2019, which will involve senior-level decision making and may involve an intentional component.
Best practices, challenges, gaps and recommendations
Considerable work has been accomplished to strengthen the linkages between public health and security and intelligence authorities. While there are memoranda of understanding and other mechanisms in place for sharing of information between public health and security authorities, there are still gaps to be addressed in exchanging information between health and security, particularly at the local level.
For example, in a response to an event or in a criminal investigation (e.g. gunshot victim), law enforcement can access patient information, if required. However, in the pre-response phase, hospitals do not share information with law enforcement routinely as information sharing is not institutionalized and standard practice.
In a case where a patient presents at a hospital with an unknown affliction, information sharing with law enforcement may be delayed or non-existent as physicians may not be considering terrorism when focused on patient care. Ultimately public health legislation allows doctors to disclose necessary information, but it is not an embedded practice and varies by jurisdiction.
While criminal and terrorist threats can clearly result in public health implications, more ongoing analysis is needed to better demonstrate how public health threats can impact national security and law enforcement interests. Continued dialogue between the Canadian public health and security and intelligence communities on roles and responsibilities will assist in clarifying this nexus.
Although work commenced in 2015 to implement a joint criminal-epidemiological investigation framework at the federal level, additional effort is required to formalize and implement a process and training program to train public health and law enforcement entities on joint investigations and/or information-sharing. The multi-jurisdictional approach to public health and law enforcement in Canada adds a level of complexity to this challenge.
Another gap identified at the federal level by law enforcement is the lack of a comprehensive threat picture. While there is a robust response for law enforcement with the National CBRNE Response Team that leverages the expertise of federal scientists and experts in assessing and responding to CBRNE threat events, law enforcement agents need better information about the kinds of threats terrorist groups are trying to use in Canada. However, information on terrorists or groups attempting to acquire precursor materials or equipment to conduct a bio-terror attack is not immediately available to law enforcement. This type of intelligence would allow a better understanding of the capability and therefore likelihood of such an attack.
Another area for improvement is information sharing, both sending and receiving. Intelligence is sometimes over classified, affecting accessibility. At other times the receiving agency may not have the knowledge or capacity to receive and handle intelligence, or the required security clearances.
Although the Public Health Agency of Canada has taken a lead role in sponsoring provincial and territorial public health authorities for security clearances, these are restricted to task-specific working groups. Additional work to integrate non-federal jurisdictions into the security clearance process needs to be undertaken but can only accomplished at a pace that is commensurate with broader increased understanding of the responsibilities and limitations inherent in sharing classified information.
R4: Medical countermeasures and personnel deployment
Joint external evaluation target: A national framework for transferring (sending and receiving) medical countermeasures (MCM) and public health and medical personnel among international partners during public health emergencies.
Level of capability in Canada
Canada has the capability to transfer medical countermeasures and public health medical personnel domestically and internationally during public health emergencies. During recent events such as the 2014 Ebola Outbreak and the recent Zika outbreak, Canada demonstrated its engagement and capability to support international efforts to reduce, where possible, the impacts of international health emergencies.
While Canada has developed significant domestic capability as it relates to the deployment of health personnel, or medical countermeasures, the complexity of international responses has driven the need to develop and review protocols, policies and plans to reflect the emerging and growing recognition that global health security has domestic health security implications.
Indicators
R.4.1 System is in place for sending and receiving medical countermeasures during a public health emergency
Medical countermeasures
Canadians expect that the federal government is sufficiently resourced and adequately prepared to respond to all manner of health threats, whether naturally occurring, accidental, or intentional. Medical counter measures (MCM) are a critical component of preparedness and response efforts. They are therapeutic products (such as drugs, vaccines, and medical devices, as defined by the Food and Drugs Act) which aim to prevent, mitigate or treat the adverse health effects of a public health event or emergency.
The Public Health Agency of Canada maintains the National Emergency Strategic Stockpile (NESS), which is a federal stockpile of health emergency assets strategically located across Canada. It is meant to be accessed as surge capacity in support of provincial and territorial governments or other federal departments when responding to emergencies that have health impacts.
Over time, the NESS has adapted to the evolving risk environment and currently maintains a number of health assets within its warehousing system to address health impacts from a variety of emergencies, ranging from flooding and wild fires, to terrorism events and disease outbreaks, such as influenza pandemics. It is also the sole provider of "niche" assets such as MCMs that address low-likelihood, high-impact public health events involving chemical, biological, radiological and nuclear agents.
Canada, through the Public Health Agency of Canada's NESS, maintains a stockpile of MCMs to provide domestic preparedness and surge capacity to the provinces and territories when their stocks have been depleted or where they do not exist. The NESS uses existing contracts, or emergency contracting methods, to procure additional MCMs for emergency use and the Public Health Agency of Canada has a previously negotiated contract to provide domestically produced pandemic influenza vaccine for Canadians at the time of a pandemic. The Canadian Armed Forces also has an influenza anti-viral stockpile it holds in the event of a pandemic for members of the Canadian Armed Forces.
To determine the type and quantity of MCMs that would best support a public health event, the Agency:
- Conducts overarching threat and risk assessments
- Performs analysis on risk mitigation
- Develops strategies for the life-cycle of the MCMs, including the procurement; storage; deployment and disposition of MCMs
These analyses are ongoing and necessary to continually evaluate MCMs as the risk environment continues to evolve in political, economic, social and environmental forums.
The Public Health Agency of Canada works closely with its Health Canada partners on legal and regulatory issues related to the stockpiling of MCMs within the NESS. This includes all activities related to approvals, product licensing, establishment licensing and compliance with good manufacturing practices (GMP). It also includes access to or use of the Special Access Program for the importation and authorized use of non-licensed medical countermeasures domestically, and the regulations on the Importation of Drugs for an Urgent Public Health Need.
The Public Health Agency of Canada routinely works with other federal departments (such as the Department of National Defence and the Canadian Food Inspection Agency) on issues related to stockpiling, coordinated procurement, and partnerships to inform the planning and procurement process.
The NESS relies on industry (pharmaceutical and medical device/assets) for the production of health emergency assets and MCMs. Based on available information at the time of an emergency, risk assessments and policy analyses would be conducted to determine the types and quantities of MCMs available on the market to respond to a public health emergency.
While Canada has negotiated the capacity to produce pandemic influenza vaccine, the capability of manufacturing MCMs during public health emergencies would be dependent on the needs of the emergency and on private industry's ability to supply the identified requirement. The Public Health Agency of Canada would work in collaboration with its procurement partners at Public Services and Procurement Canada to inquire on market availability to provide or produce required MCMs during a public health emergency.
There are contracts for certain MCMs that allow for expedited amendments to contracts or, where contracts do not exist, there are emergency contracting procedures in place. For pandemic influenza, there is a contract with a domestic manufacturer to produce vaccine. The manufacturer will be responsible for distributing the vaccine to provinces and territories. The Government of Canada, where appropriate, will collaborate with provinces and territories towards joint or coordinated procurement efforts for specific MCMs, including antivirals.
The Canadian Pandemic Influenza Plan: Planning Guidance for the Health Sector (CPIP) specifically addresses countermeasures. The CPIP provides planning guidance for the health sector for pan-Canadian pandemic influenza preparedness and response. The Plan consists of a main body that provides strategic guidance, and a framework for pandemic preparedness and response. It also contains technical annexes that provide detailed operational advice, technical guidance and tools and checklists. The vaccine Annex of the CPIP addresses risk management considerations.
Federal government policies for deploying medical countermeasures domestically
The NESS routinely deploys assets domestically to address health emergencies. The deployment of MCMs must comply with the transportation requirements set out in Canada's Good Manufacturing Practices (GMP) Guidelines, whether shipped domestically or internationally. The NESS has a series of standard operating procedures in place for the deployment of assets, including those requiring cold chain maintenance and staff who are recertified under the Good Manufacturing Practices regulations on an annual basis.
Security concerns regarding the MCM stockpiles are identified in threat and risk assessments of NESS GMP-certified facilities. Canada now has two fully functioning GMP-certified facilities. When shipping/receiving or procurement contracts are put in place, security assessments are completed that identify and/or address security risks associated with specific MCM acquisition or deployments.
Canada supports domestic responses through the deployment of MCMs or other health assets from the NESS upon request from provinces or territories when they have identified a need for surge capacity or if resources are unavailable. The Public Health Agency of Canada also maintains an Operational Framework for Mutual Aid Requests. The Framework currently accommodates requests from health professionals, but it will be expanded to consider requests for assets in health emergencies. Assets would include MCMs from federal, provincial or territorial stockpiles. The requests are processed through a single-window notification system in the Health Portfolio Duty Officer standard operating procedures.
Currently, MCMs are located within two GMP-certified facilities in the National Capital Region of Canada. The feasibility of and operational capability to preposition MCMs in strategic locations to enable increased response capability is currently under consideration. The NESS uses commercial temperature controlled courier companies on standing offer agreements with the Government of Canada, to transport MCMs across Canada under strict environmental conditions.
In the absence or lack of availability of commercial transportation, the Public Health Agency of Canada may call on federal partners to assist in the event that rapid deployment of an MCM across Canada is required. For example, the Royal Canadian Mounted Police may be called upon to activate a memorandum of agreement or alternately, an official request for assistance may be submitted to the Department of National Defense for the use of federal aircraft.
The development of agent-specific MCM deployment plans and guidance documents are currently being considered in collaboration with provinces and territories. These plans would help to solidify the requirements for the request, deployment, and use of the various MCMs within the NESS when deployed to provincial and territorial health sector partners.
The NESS provides logistics support in the shipment of health assets. However, the delivery and receipt of MCMs is a collaborative federal, provincial and territorial governmental activity. The NESS tracks the deployment from the national stockpile to the identified provincial or territorial authority. Distribution of MCMs beyond the initial shipment is managed by provincial/territorial health authorities.
Provincial and territorial governments also conduct their own threat and risk assessments and plan for health emergencies in their own jurisdictions accordingly. These plans include the use of MCMs where risks have been identified. As a result, provincial and territorial governments not only have anti-viral stockpiles, but other types of MCMs that are relevant to their regional risks. For example, provinces and territories with nuclear power plants stock radiation-appropriate MCMs for their populations.
In Canada, pandemic vaccine is distributed by the manufacturer. In a pandemic, the Public Health Agency of Canada would likely play a role in tracking the status of anti-viral stockpiles. This is a role the Agency assumed during the H1N1 pandemic crisis in 2009.
Federal government policies for deploying medical countermeasures internationally
The deployment of MCMs internationally is assessed on a case-by-case basis and involves extensive consultation with federal partners who have an international mandate. This includes Global Affairs Canada which has a priority to contribute to international peace, security and humanitarian assistance through renewed leadership and constructive international engagement.
In the event that a request for assistance is received and international deployment is considered feasible, the Public Health Agency of Canada would conduct an analysis to consider a number of legal, logistical, domestic risk and regulatory concerns. These include, but are not limited to:
- Legal or policy barriers to the deployment of MCMs internationally
- Legal authorities and mandate in existing deployment mechanisms
- Liability concerns regarding the international deployment of MCMs
- Replenishment costs or funding issues including deployment costs
- Importation and Exportation requirements (in consultation with Canada Border Services Agency)
- Logistical issues such as cold-chain maintenance
- A review of domestic risks is completed to determine if an international response unreasonably reduces domestic health security levels
The use of commercial shipment and delivery methods for the international deployment of MCMs is the preferred route. However, the use of military or other available transport methods may be considered depending on the circumstances of the request and logistics needs.
International collaborations related to medical countermeasure deployment
The Agreement between the Government of Canada and the Government of the United States of America on Emergency Management Cooperation also has increased cross border cooperation on a full range of emergency management and national security issues. Dialogue on the sharing of MCMs is ongoing.
Provincial governments in Canada have regional agreements among themselves and with neighbouring U.S. States that speak to broader mutual assistance issues that may include the exchange or sharing of MCMs. Opportunities to explore areas for collaboration are discussed at the international level through:
- The Medical Countermeasures Consortium, a partnership between defence and public health departments of the United States, the United Kingdom, Australia, and Canada
- The Global Health Security Initiative, involving the G7 countries-France, Germany, Italy, Japan, United States, United Kingdom, and Canada-plus Mexico, the European Commission, and the World Health Organization
- Bilateral partnerships (for example with the United States)
Sharing of information and coordination of acquisition are ways that countries work together to find economies of scale.
Internationally, even though the NESS does not have an international mandate, the Public Health Agency of Canada works with its Government of Canada partners in the deployment of assets abroad and a series of key procedures and decision points would need to be considered prior to release of an MCM from the NESS. As mentioned previously, this may include a review of such issues as policy and legal authorities, importation/exportation requirements, and logistics.
The Convention on Assistance in Case of a Nuclear or Radiological Emergency sets out an international framework for prompt international assistance and support in the event of nuclear accidents or radiological emergencies. It requires the receiving country to invoke the Convention and each State Party then decides whether it can offer their available experts, equipment and other material (as well as, scope and terms) for providing assistance in the case of a request.
Receiving medical countermeasures from international partners
There are regulatory mechanisms in place in Canada that allow for the sale and importation of drugs not licensed in Canada but licensed by credible regulatory agencies/bodies. Decisions to allow for sale and importation would be based on a risk/benefit analysis.
The Public Health Agency of Canada would work with its regulatory partners at Health Canada to conduct an analysis to determine whether receiving MCMs from international partners would be feasible. This would include, but not be limited to, consideration of legal barriers or authorities, as well as current policies related to the use of the Special Access Program for importation and authorized use of non-licensed MCMs domestically.
Lessons learned from international medical countermeasure deployments
Continuous improvement methodologies and lessons-learned evaluations are a routine part of the Government of Canada's response to real-world events or crises. Recent events have identified the following specific improvements for consideration in the deployment of MCMs:
- Federal departments should work together in advance of international events to improve coordination and to pre-identify and understand legal, statutory and policy authorities.
- Policies and regulatory reviews or directives related to the deployment and use of unapproved or unlicensed MCMs should be clear.
- Importation and exportation regulations and requirements should be commonly understood and exercised to ensure processes are clear and well-coordinated.
- Logistical processes should be identified in advance of an incident, in particular where it concerns the maintenance of cold chain standards for international shipping to locations where cold chain may be at risk.
Examples of exercises related to domestic and international plans for sending and receiving MCMs that have been conducted in the past year are listed below.
- In April 2016, PHAC sponsored the Health Portfolio Workshop on Domestic and Cross-Border Emergency Health Mutual Assistance. The workshop brought together federal departments and agencies to examine the international plans involved in cross-border sending and receiving surge capacity, including MCMs. The exercise identified a broad range of plans, protocols, and agreements that could be used to request and/or provide MCMs. The exercise increased knowledge across both emergency management and health authorities.
- In June 2016 during Exercise Pacific Quake, the exercise examined domestic plans for requesting medical surge (including MCMs). The exercise also examined contingency planning if domestic stocks were not sufficient how bilateral requests with the US and or international requests could be made.
- In April 2017, the federal government participated in Staunch Maple 17, which examined provincial, federal, cross-border and international requests for MCMs.
R.4.2 System is in place for sending and receiving health personnel during a public health emergency
Plans for sending and receiving health professionals during emergencies
Domestically, provinces and territories are responsible for the delivery of health care within their jurisdictions. Provinces and territories maintain a variety of bilateral, regional, national and international agreements to share human resources (including health professionals) during emergencies.
One of the key national-level agreements that facilitates the deployment of health professionals is the Federal/Provincial/Territorial Memorandum of Understanding on the Provision of Mutual Aid in Relation to Health Resources During an Emergency Affecting the Health of the Public, which was signed by all federal, provincial and territorial Ministers of Health in 2009. Following the signing of this memorandum of understanding, the Operational Framework for Mutual Aid Request was developed as a tool to help consolidate and coordinate offers of assistance from multiple jurisdictions (federal, provincial/territorial, and the Canadian Red Cross). Although originally created for physicians and nurses, the tool is flexible and can expand to identify other healthcare professionals. To date, the Operational Framework for Mutual Aid Request has been activated three times:
- June 2013 (Calgary Floods)
- June 2015 (Saskatchewan Wildfires)
- May 2016 (Fort McMurray Fires)
Ultimately, the receiving jurisdiction maintains full authority on addressing the licensing and registration of healthcare professionals. Provinces and territories have measures in place to quickly grant special permissions to professionals to deliver care within their jurisdictions during emergencies.
When provinces require surge capacity to support outbreak investigations or other emerging public health events, they are able to request support from the federal government for technical experts. These can include epidemiologists, environmental health officers, quarantine officers and medical advisors. The Public Health Agency of Canada has programs dedicated to building Canada's public health workforce, including the Canadian Field Epidemiology Program, which requires all program participants to complete at least one deployment to support emergency response activities (see section D4: Workforce development).
In 2016, the federal Health Portfolio completed a thorough review of both domestic and international personnel deployment policies, procedures and practices. The Health Portfolio Mobilization Strategy for Event Response was developed to highlight current system strengths and areas of improvement. The Strategy identifies 27 action items, many of which are currently being implemented. For example, the Health Portfolio has developed a mandatory training plan for employees wishing to participate in deployments, with specific requirements for both domestic and international activities.
International deployment of public health and medical personnel
The federal Health Portfolio is in the process of developing a policy for external mobilizations that will apply to both domestic and international personnel deployments during emergencies. The Health Portfolio's mandate to respond to international emergency response activities is derived in part from the Public Health Agency of Canada Act (2006), which recognizes that the Government of Canada wishes to foster collaboration in the field of public health with foreign governments and international organizations as well as other interested persons or organizations.
This includes broad authorities to strengthen Canada's international coordination with key global partners and support public health interventions focused on disease control and emergency response. International mobilizations for event response are also part of Canadian obligations under the International Health Regulations (2005).
For many years, the federal Health Portfolio has responded to requests for assistance received from government and non-government partners including Global Affairs Canada, the U.S. Centers for Disease Control and Prevention, and the World Health Organization Global Outbreak Alert and Response Network.
Since 2011, the Health Portfolio has experienced an increase in the number of requests for assistance received from international partners seeking surge capacity to respond to events with public health consequences, including disease outbreaks and natural and human-caused disasters. This trend is predicted to continue as risks to public health security are increasing due to higher volumes of international travel and trade, and to the public health impacts of climate change, urbanization and other factors.
The Health Portfolio's international practice has most often involved mobilizing technical experts in the fields of epidemiology, laboratory and diagnostics, infection prevention and control, emergency management, biosafety and biosecurity, quarantine and border health.
The Health Portfolio also has limited capacity to provide health care professionals. When employees are mobilized internationally, they are always deployed through international partners who provide security and logistical support on the ground and in the field.
Canada is part of several regional/international personnel deployment agreements, including:
- Global Outbreak Alert and Response Network
- Pan American Health Organization
- CANUS Civil Assistance Plan, which allows for drawing upon U.S. medical assets to assist the Department of National Defense
Other pandemic preparedness plans or other emergency preparedness plans that address personnel deployments include:
- The Federal, Provincial and Territorial Public Health Response Plan for Biological Events. Emergency Management authorities facilitate and support coordination of responses to public health events by addressing issues regarding mutual assistance or aid (e.g. via the Operational Framework for Mutual Aid Requests).
- Department of National Defense response is through CONPLAN LASER for pandemic, CONPLAN RUBICON for Chemical, Biological, Radiation and Nuclear response.
- The Canadian Pandemic Influenza Preparedness (CPIP): Planning Guidance for the Health Sector is the federal, provincial, and territorial guidance document that outlines how jurisdictions will work together to ensure a coordinated and consistent health-sector approach to pandemic preparedness and response. CPIP consists of a main body, which outlines overarching principles, concepts, and shared objectives, and a series of technical annexes that include more detailed operational plans on specific issues.
There are many non-governmental organizations operating within Canada that regularly contribute to international responses to emergencies with health consequences. For example, the Canadian Red Cross provided emergency social services during the Alberta wildfires and has deployable field hospital capability.
Best practices, challenges, gaps and recommendations
Governments at all levels in Canada continue to work to protect the health of Canadians by adapting to the evolving risk environment that may impact public health. To achieve this, the Public Health Agency of Canada has adopted a flexible approach to conducting risk and threat assessments; analyzing risk mitigation; and developing strategies for procurement, deployment and disposition of medical countermeasures.
There is also recognition that collaboration is an essential component to sending and receiving MCMs during a public health emergency. Given that the role of the Government of Canada is to provide leadership and guidance during public health emergencies, the Public Health Agency of Canada continues to foster relationships both domestically and internationally. This is demonstrated through its frequent interactions with federal, provincial and territorial governments, and other federal partners, such as Health Canada.
Issues for discussion include licensing, good manufacturing practices and the access or use of the Special Access Program. The Agency also exchanges internationally with the Quadrilateral Medical Countermeasures Consortium, the Global Health Security Initiative and bilaterally (with the United States, for example) on methods for sharing information and coordinating acquisitions.
Although international requests are assessed on a case by case basis, responding to global health security events can be complex, and in an increasingly interconnected world, these events could have domestic public health implications. While there is a robust process in place for responding domestically with MCMs, there are areas for improvement in sending and receiving MCMs internationally.
The Government of Canada is also seeking to strengthen personnel deployments by updating processes, tools, agreements and policies through the implementation of the Health Portfolio Mobilization Strategy for Event Response. The overall goal of the Strategy is to ensure Canada has the ability to deploy trained personnel in a timely manner based on evidence-informed decision-making processes. The Strategy could be considered a best practice.
In an effort to gain a better understanding of how international response with MCMs could have domestic public health implications, further examination of the current challenges that may limit MCMs being sent or received internationally should be considered. This could include seeking authority to remove barriers to rapid deployment of MCMs internationally, or a pre-identification of various legal, logistical or regulatory issues. It could also include an examination of existing international agreements such as the Agreement between the Government of Canada and the Government of the United States of America on Emergency Management Cooperation to determine if concepts could be leveraged with other international partners.
R5: Risk communication
Joint external evaluation target: State Parties should have risk communication capacity that is a multi-level and multi-faceted real-time exchange of information, advice and opinion between experts and officials or people who face a threat or hazard to their survival, health or economic or social well-being so that they can take informed decisions to mitigate the effects of the threat or hazard and take protective and preventive action. It includes a mix of communication and engagement strategies like media and social media communication, mass awareness campaigns, health promotion, social mobilization, stakeholder engagement and community engagement.
Level of capability in Canada
Canada has a complex health care system that involves multiple levels of government, multiple jurisdictions and numerous stakeholders. To ensure seamless risk communications, Canada uses a variety of mechanisms to create coordinated, clear and timely flow of information to Canadians in a health emergency. Each level of government develops and maintains networks that support information coordination and dissemination, taking into account the cultural and regional diversity of Canadians.
Canada continues to build its risk communications capacity at all levels of government. Various networks and working relationships are in place that can be called upon in a federal health emergency. These groups also share lessons learned, exchange best practices, and organize workshops and exercises.
As a result, organizations at the federal, provincial, territorial and municipal levels are able to coordinate, prepare for, and respond to a variety of emergency events related to health, including natural disaster, terrorist attack, outbreak and/or epidemic.
Canada takes advantage of its population's affinity for electronic and mobile communications by using multiple social media platforms and web- and mobile-based applications to communicate with Canadians daily on a variety of issues. These existing communications channels can be used in emergency situations. At the same time, Canada ensures that a variety of communication mechanisms are used to reach populations with limited internet access and particular accessibility requirements. Diverse communication needs, such as language and cultural variances, can be accommodated.
The responsibility for communicating with Canadians during an emergency is shared by the federal, provincial, territorial, and municipal governments. Within the federal government, Health Canada and the Public Health Agency of Canada are responsible for the majority of national health communication activities. The Communications and Public Affairs Branch of Health Canada supports the communications efforts of both organizations. Within the Branch, the Risk and Emergency Communications team coordinates risk and emergency communications preparedness, response and training within the Health Portfolio.
Indicators
R.5.1 Risk communication systems
National risk communication plans and multiagency coordination
National context
Public Safety Canada houses the Government Operations Centre, which is responsible for coordinating the Government of Canada's response to an emergency. When the Government Operations Centre is activated, federal departments and agencies feed into it to support the federal response, including the Health Portfolio Operations Centre. When an emergency is health-related, the Health Portfolio Operations Centre leads the coordination of the federal response.
In situations where a health-related emergency is limited to a specific geographic region, the relevant provincial, territorial or municipal government leads the response. The lead government will engage other levels of government as required, depending on the nature of the event. Provincial or territorial governments are responsible for disseminating information directly related to health care delivery.
For all federal health emergencies, and situations that involve multiple jurisdictions, the Health Portfolio leads the coordination of communications with the public. Relationships with provincial and territorial governments are used to ensure a coordinated response. Federal networks, such as the Public Health Network Communications Working Group, regularly bring together federal, provincial and territorial government representatives to exchange information and to ensure effective and well-coordinated health-related communications across the country.
Numerous emergency response plans outline the roles of Canadian government departments and agencies, as well as the roles and responsibilities of provincial, territorial and municipal governments.
The Federal Emergency Response Plan identifies regional roles, responsibilities and networks, states that private and non-governmental organizations may be engaged as necessary, and includes a section dedicated to communicating with the public.
Federal Health Portfolio
Canada's Health Portfolio Emergency Response Plan outlines the communications function in Annex F: Health Emergency Risk Communications Protocol. The Plan and the Emergency Risk Communications Protocol are used to implement risk communication practices during health emergencies. They also contain information on protocols, roles and responsibilities, and the importance of addressing risk perception in a culturally appropriate way.
When the Health Portfolio Operations Centre is activated, a communications professional well-versed in risk communications is deployed to the Centre to advise on communications procedures, coordinate information and messaging with other federal departments and agencies, and develop communications plans and products.
The Risk and Emergency Communications team is responsible for providing risk communications advice to enhance risk communications capacity at Health Canada and the Public Health Agency of Canada. As the team is small, in times of emergency, communications professionals from elsewhere in the Communications and Public Affairs Branch may be assigned to the team to provide surge capacity. There is currently no list of employees identified for surge capacity during an emergency, however the Branch will develop a list of individuals available for mobilization to support communications in an emergency.
In addition to the risk communications systems in place through the Health Portfolio Emergency Response Plan and the Health Portfolio Operations Centre, Health Canada and the Public Health Agency of Canada have developed the Toolkit for Health Risk Communications, as a resource to help organizations assess the nature of risk and to develop appropriate communications approaches.
The Health Portfolio also maintains a toll-free number for information on federal services related to health (1-866-225-0709 and info@hc-sc.gc.ca). Members of the public may use this line in emergency and non-emergency situations. The federal government runs 1 800 O-Canada for questions from the public about all federal services, programs, and information, including those related to health. When questions related to the mandate of the Health Portfolio are received, callers are provided with federal information and may be redirected to the appropriate department or agency. Both services provide references to emergency services and first responders.
The Government of Canada uses expedited processes for urgent approval of communications materials, such as media requests in emergency situation.
Provinces and territories
Similar services exist within provincial and territorial governments. Some examples include Alberta's 811 Health Link and Mental Health Help Line and Ontario's Telehealth Ontario, a toll-free number that allows members of the public to contact a registered nurse 24 hours a day, seven days a week.
At the provincial and territorial level, as at the federal level, an all hazards approach is taken regarding emergency planning, including communications. While communications-specific plans may not exist, communications is part of overarching emergency response plans. For example, the Manitoba Emergency Plan includes protocols for communicating with the public, stakeholders and other levels of government.
All public health plans and guidance documents contain a communications component. Alberta's Pandemic Influenza Plan explains how public communications will be coordinated among organizations involved in the response, including a list of communications roles and responsibilities for the lead agencies and their partners. It also designates spokespeople and provides some guidance on communications tools.
Staffing and budgets for risk communication
Within Health Canada, there is a dedicated budget to sustain communications functions, which is reviewed regularly. However, there is currently no plan or process in place to ensure effective surge capacity for risk communications. While Communications and Public Affairs Branch staff are asked to voluntarily participate in short-term event responses, not all staff are trained in risk communications and the available resources are not sufficient to ensure coverage over a long-term emergency situation. To address this, the Branch is developing a list of communications professionals in the Health Portfolio who have the appropriate training, experience and interest to provide surge capacity in the event of an emergency.
Provincial and territorial governments have communications professionals who serve the needs of their ministries, including health. These services may be within individual ministries or centralized. For example, Manitoba has a centralized communications division, Communications Services Manitoba, which coordinates public communications during emergencies on behalf of all departments. In Ontario the Ministry of Health and Long-Term Care houses its own communications division.
Risk communication training and exercises
Within the Government of Canada, the Health Portfolio Operational Subcommittee is the governance committee that oversees and approves updates to the Health Portfolio's plans. The communications function is well represented on this committee.
Government of Canada emergency plans are tested regularly via emergency exercises, but not all plans are tested every year. Communications is generally included in these exercise activities; however, the implicated organizations are not always able to fully participate due to limited resources. For this reason, exercise results may not always fully reflect the current capacity of communications teams to respond. After-exercise reports identify areas for improvement that inform plans and processes for future events.
Incident management system training is available to all Health Portfolio communications staff. The Risk and Emergency Communications team delivers Emergency Risk Communications Training on a regular basis. This one-day training-open to all employees of the Public Health Agency of Canada, Health Canada and the Canadian Food Inspection Agency introduces staff to risk best practices in communications using one of three emergency-based fictional scenarios:
- infectious disease
- food-borne illness
- radiological/nuclear incident
R.5.2 Internal and partner communication and coordination
Government-to-government communications
In emergency situations, the Government of Canada works across its departments and agencies to deliver a whole-of-government, all-hazards approach to an event response, including communications. Federal government departments and agencies work with provincial and territorial governments to ensure that emergency responses are consistent, well-coordinated, effective and timely. Provinces and territories work with municipalities as appropriate. Working groups and networks, such as the Public Health Network Communications Working Group, ensure that communications with Canadians on public health issues are consistent and well-coordinated by bringing together federal, provincial and territorial government representatives. These networks can be tapped to support an emergency response
The communications position within the Health Portfolio's Incident Management System is responsible for coordinating communications during an emergency. This capacity includes internal communications within Health Canada and PHAC. Internal communications mechanisms include a daily newsletter to all staff, Health Canada TV, which broadcasts on screens in PHAC and Health Canada buildings, and targeted email messages from senior executives, such as the Deputy Minister of Health Canada and the President of PHAC. Other mechanisms specifically designed for emergencies are outlined in business continuity plans.
The incident management system is also used to coordinate communications with other federal departments and agencies, provincial and territorial partners, and other major players. If the emergency goes beyond a health response, the Health Portfolio Operations Centre feeds into the response led by the Government Operations Centre.
The communications lead filling the communications function in the incident management system also coordinates communications among national stakeholders and response agencies during an emergency. Communications with provincial and territorial partners is coordinated through the Public Health Network Communications Working Group.
When an emergency situation is taking place within a First Nations or Inuit community, the First Nations and Inuit Health Branch of the Department of Indigenous Services Canada coordinates information and action required through its regional contacts, who are in direct contact with community representatives (such as Chiefs, band members, and nursing station personnel within community nursing stations). Health Canada maintains communications with Indigenous organizations, such as the Assembly of First Nations and Inuit Tapiriit Kanatami during an emergency.
Provincial and territorial governments have developed individual processes and networks to support their coordination with federal and municipal governments. Communications Services Manitoba, for example takes part in a regular working group with regional health authorities and provincial health organization communicators. Communications Services Manitoba also has connections with the Association of Manitoba Municipalities, which works with the province during emergencies to circulate information to its members.
Internationally, the Health Portfolio Operations Centre and the Government Operations Centre coordinate communications with stakeholders during emergencies. The Risk and Emergency Communications team also maintains membership in the Global Health Security Action Group, which includes members from the G7 and Mexico. This network has been used in the past when developing communications within the international context (e.g. Zika virus and the 2010 Vancouver Olympics) and could serve that function again. A trilateral Declaration of Intent to Coordinate Health Emergency Public Communications between Canada, the U.S. and Mexico was also signed under the North American Plan for Animal and Pandemic Influenza.
Communication with the health system and civil society
When appropriate at the federal level, civil society and representatives from provincial and territorial governments (health systems) are included in response coordination via the Health Portfolio Operations Centre and/or communications-specific networks. The Public Health Network Communications Working Group can be called upon in times of emergency to coordinate effective communication and ensure consistent messaging from government authorities. Existing networks and working groups are also used to connect with audiences and develop relationships with different non-governmental organizations.
For communicating to civil society, the Health Portfolio Operations Centre has, in the past, included non-governmental organizations, such as the Red Cross, in coordination activities. In times of emergency, existing relationships with non-governmental organizations can be leveraged to support communications and the Government's emergency response. For example, during the Ebola outbreak in West Africa, federal communicators worked closely with the Canadian Red Cross to coordinate communications related to Canada's response and national preparedness should Ebola come to Canada.
The federal government works with all jurisdictions across the country to ensure that messages are consistent and credible. This collaboration is conducted through teleconferences, briefing sessions, face-to-face meetings, and behind the scenes coordination meetings.
Provincial and territorial governments have protocols and mechanisms for communicating with hospitals, the health care sector, and other stakeholders, such as non-profit organizations. For example, Manitoba has a well-developed system that uses a variety of mechanisms to ensure effective risk communications and response. Some elements of this system include:
- A duty officer in the Office of Disaster Management in Manitoba's Department of Health ensures timely information flow with impacted health facilities, health organizations, and emergency management organizations during significant public health events and/or other health emergencies to ensure that information is shared broadly with government, community and regional health system leaders. The duty officer also makes use of relationships built through the health system's Disaster Management Network, which brings together regional and provincial level health emergency managers, to share information, ensure joint planning where applicable and troubleshoot issues of concern.
- Medical health officers, who work collaboratively with each other and with regional and provincial communications professionals to ensure timely and effective communications with communities and the public on public health issues.
- Communications Services Manitoba, which participates in the Emergency Communicators Roundtable, a group of stakeholder communications staff involving civic, education, law enforcement, health care, and federal and provincial communicators, who meet regularly to share information and build networks that can be accessed during emergencies.
Relationships between all levels of government and non-governmental organizations and private partners exist across Canada and are based on local issues and governance structures.
At the federal level, while there is no formal mechanism to coordinate communication with the private sector during an emergency, the Health Portfolio Operations Centre Incident Management System can coordinate with private sector organizations through stakeholder engagement and regional coordination liaisons. In these situations, communications staff work in parallel with the private organizations to ensure coordinated messaging. This has been done in the past as needed.
Communication exercises
The Public Health Agency of Canada and Health Canada participate in exercises with other government departments and levels of government, both domestic and international. Exercises have also included private organizations in the past. For example, Exercise Intrepid 2015 was a two-day exercise that involved a simulated nuclear incident in New Brunswick. Thirty private and public-sector organizations at the provincial, municipal and federal levels participated in this exercise.
Beyond exercises, the Public Health Agency of Canada has participated in multiple events that have required expanded communication coordination. The most recent events were the Syrian Refugee Response, Ebola, the Alberta Wildfires, and floods.
Avoiding inconsistent or inappropriate messages
The Public Health Agency of Canada works with other federal government departments and provincial and territorial governments when developing emergency plans, most of which have a communications component. Examples include Canadian Pandemic Influenza Preparedness: Planning Guidance for the Health Sector, which has a communications and stakeholder annex, as well as "dashboards" that provide more specific direction for communications staff. However, these are not dedicated communications response plans; they are communication guidelines and/or protocols included in an overarching emergency response plan. The Public Health Agency of Canada does not work with stakeholders from the private sector or non-governmental organizations to create communication response plans.
Indigenous Services Canada consults Indigenous communities and organizations, such as the Assembly of First Nations and Inuit Tapiriit Kanatami, to develop culturally relevant communication materials, including emergency communication materials.
Communications is done differently at each level of government and in each jurisdiction. As a result, there is a risk that contradictory or inconsistent information could be released during an emergency. However, this is not common as most communications personnel across Canada understand the importance of coordination and cooperation for effective risk communications during emergencies.
R.5.3 Public communication
Communication resources and spokespersons during emergencies
All Government of Canada departments and agencies communicate with the public to provide services and to inform Canadians of the work being done within their mandate. These activities are guided by the Government of Canada Policy on Communications and Federal Identity and the associated Guideline on the Management of Communications, which clearly states that the federal government must designate spokespeople and work proactively with the media to promote public awareness and understanding of government policies, programs, services and initiatives.
Both the Minister of Health and the Chief Public Health Officer of Canada are spokespeople during health emergencies. The Chief Public Health Officer has a legislated responsibility to communicate to Canadians about health impacts and risks in times of emergency. Other Government of Canada spokespersons, such as the Minister of Health and Indigenous Services Canada's Chief Medical Officer of Public Health, may speak publicly on behalf of the Government during an emergency, providing information on what steps the government is taking to address the emergency. The Minister of Health and the Chief Public Health Officer have official Twitter accounts to facilitate direct and timely communication to the public on a variety of public health issues.
Health Canada's Communications and Public Affairs Branch provides communications advice and services to Health Canada and the Public Health Agency of Canada. The Branch, with approximately 300 employees, provides a variety of services, including strategic communications, media relations and monitoring, public consultations, regional communications, internal communications, creative services, and digital communications. This includes, social media (Twitter, Facebook, LinkedIn), web content, print publications, advertising, and marketing. The Branch also takes advantage of the many communication channels and networks available across the Government of Canada.
Each province and territory manages its communication resources differently. However, like their federal counterparts, they use a variety of mechanisms to communicate with the public. These include social media, web sites, and designated spokespersons, such as Chief Medical Officers of Health. Two examples of provincial tools used to communicate with the public in emergencies are Manitoba's Emergency Measures Organization media alerts and Alberta Emergency Alerts, which include traditional broadcasting media, road signage, and various web-based applications (RSS feeds, social media, and the Alberta Emergency Alert Application).
Language and cultural relevancy
In accordance with the Government of Canada's Policy on Communications and Federal Identity, all communications to the public from the federal government must be available in English and French. The Government of New Brunswick also produces all of its materials in its two official languages, French and English, and the Government of Nunavut produces materials in English, French, Inuktitut and Inuinnaqtun.
Where appropriate, materials are also developed in other languages, including Indigenous languages and languages of target populations. Adapting public health messaging according to the needs and contexts of the target audience is done selectively, as required.
Additionally, as with all communications to the public, Government of Canada institutions must respect the Canadian Multiculturalism Act which states that all federal institutions must "generally, carry on their activities in a manner that is sensitive and responsive to the multicultural reality of Canada." In keeping with this legislation, public communications always consider the diverse cultural realities of the Canadian population, and communications products are developed accordingly.
At all levels in Canada, communicators follow communications best practices to ensure information is clear and easy to understand, and tailored to specific audiences.
At the federal level, campaigns and initiatives may use public opinion research and consultations to inform plans so that products meet the needs of Canadians. Communications strategies developed on a case by case basis may include proactive outreach on specific topics such as immunization, antimicrobial resistance, and Zika. These strategies use a variety of tactics and mechanisms to share information with Canadians proactively and on a sustained basis.
Developing evidence-based communications and new strategies
Health Canada and the Public Health Agency of Canada are evidence-based organizations and share with the public the evidence that has informed their decisions or that may help Canadians make decisions about their health. This is in line with the Government of Canada's overarching commitment to transparency and open government. Similarly, the Government of Canada seeks innovative approaches to communications to deliver effective and targeted messaging.
At the federal level, for select health-related marketing campaigns, research to determine message reach and understanding is conducted. For example, media monitoring, and web and social media metrics are often used to determine a campaign's reach. Public opinion research and focus groups are also used to complement these metrics, measure audience understanding and inform future communications plans and activities. Data are also obtained from baseline surveys and post-campaign evaluations such as the Government of Canada's mandatory Advertising Campaign Evaluation Tool, which is required for all advertising campaigns with a media component of $1 million or more, and is used to track audience recall, understanding and response.
During emergencies, the federal government provides regular media briefings and updates through traditional and social media. Updates may include social media posts, web postings, news releases, and announcements, press conferences and technical briefings. If the emergency includes multiple departments and agencies or crosses jurisdictions, Government of Canada communications are coordinated through Public Safety Canada.
Within provincial and territorial jurisdictions, the extent of media research varies and can be resource and issue dependent. However, all jurisdictions use social media to varying degrees to communicate to the public.
The Public Health Agency of Canada uses lessons learned and evidence-based content in its Emergency Risk Communications training to help employees understand what communications methods are most effective at influencing behaviour during emergencies. Instructors use case studies and real-life examples to demonstrate why existing processes may need to be improved and/or modified to support more effective risk communications during an emergency.
This type of information is also shared with employees via one-on-one consultations, and through risk communication tools and other guidance documents. Communications personnel also participate in the Government Emergency Communications Network. This network of communications professionals from across the federal public service work to enhance emergency and risk communications capacity and to build knowledge across departments.
The Risk and Emergency Communications team works with, and provides training to, a variety of Government of Canada organizations responsible for risk communications. The team has also provided training to international organizations and governments, including the Pan American Health Organization and the Government of Guinea. The team shares best practices and lessons learned with communicators from the provinces and territories via the Public Health Network Communications Working Group and with other federal government departments via the Government Emergency Communicators Network.
The Government of Canada works diligently to ensure that Canadians always have the most up-to-date and accurate information possible. Through dedicated media monitoring and public environment analysis, communications professionals are able to adapt and update messaging as needed. In emergencies it can be more difficult to address erroneous information in a timely and consistent manner. This requires dedicated resources and the ability to mobilize surge capacity. In an emergency the Health Portfolio can draw upon existing resources to provide additional traditional and social media monitoring and analysis that enables communications advisors to change messaging to meet changing public needs.
R.5.4 Communication engagement with affected communities
Social mobilization
Social mobilization in Canada-in the context of health promotion and emergency preparedness and response-draws on the techniques and practices of social marketing and risk communications to engage and motivate stakeholders to take action for social or behavioural change. Governments in Canada at all levels have considerable experience using these techniques on a variety of public health issues, such as:
- Stop smoking
- Food safety
- Seasonal flu
- Childhood vaccination
- Emergency preparedness
Health promotion and community engagement are considered fundamental components of public health in Canada. All levels of government have social mobilization, health promotion and/or community engagement groups or experts, such as British Columbia's Government Communications and Public Engagement Team, Alberta's Community Engagement and Communications team, and Health Canada's Risk and Emergency Communications Division. During an emergency, communications specialists in these fields are available across the country to support response activities and to work closely with media officers and designated spokespersons as part of the development and implementation of communications strategies, including those with social mobilization goals.
At the federal level, Health Canada and PHAC make training in risk communication processes, practices and principles available to all Health Portfolio employees, and these techniques are integrated, when appropriate, into federal public health communications, including during emergencies. Indeed, the Health Portfolio's Framework for Strategic Risk Communications recognizes that communication is the most powerful influence on people's risk decision-making and behaviour and is therefore essential to enabling people and organizations to manage risks effectively. The Framework outlines an approach that uses social mobilization principles to engage people to think about and understand their situation and to motivate them to act to achieve specific goals. The Framework focuses on the interests, priorities, values and perceptions of stakeholders who face the greatest risk and is grounded in sound scientific information and transparent communication.
In most jurisdictions, emergency incident management structures include a communications function. If social mobilization, health promotion or community engagement expertise is required, the communications section will bring in relevant specialists to plan and implement appropriate activities. The Federal, Provincial, and Territorial Public Health Response Plan for Biological Events-which covers events that require a national response-includes a public health communications group as part of the emergency governance structure. This group would have access, during a response, to a wide range of communications specialists from provinces, territories and federal departments.
Public health communications specialists at all levels of government are connected and share lessons learned with each other through formal and informal channels, including conferences, webinars, and the Public Health Network's Communications Working Group. Recently, Public Health Ontario hosted a Grand Rounds webinar where municipal public health agencies shared best practices in the use of social marketing techniques and social media to motivate new parents and young people at risk for mumps to adopt behaviours to avoid infection, including vaccination.
"Social capital," the relationships and networks that facilitate collective action, is essential to all effective public health communications. Direct communication with affected communities in Canada is primarily led at the local level by regional health authorities, community health centres and other community-based organizations. Provinces and territories build relationships and networks within their jurisdictions: health officers working on the front line know their clients can identify and connect different stakeholders quickly and are crucial channels of communication and information.
Federal departments also build these relationships and networks, although not directly with communities (except in the case of Indigenous Services Canada which works directly with First Nations and Inuit communities). The federal government engages regularly with intermediary stakeholders, such as provincial and territorial public health authorities and communicators, academics, and accreditation and regulatory bodies for medical practitioners. This ongoing engagement supports collaboration between levels of government during an emergency, and ensures targeted and direct messaging reaches communities when needed. The Health Portfolio also maintains a database of some 6,000 stakeholders across Canada who can engage community leaders in a variety of communications initiatives. As part of the response to the opioid crisis in Canada, for example, the Health Portfolio used its networks to create and disseminate health information to leaders of affected communities who could then incorporate it into local social mobilization activities.
Indigenous Services Canada works directly with many First Nations and Inuit communities to ensure access to health care, to engage communities, and to promote health. They build on-the-ground networks and connect these to broader provincial, territorial and federal networks. When a First Nations community declares a state of emergency or requires assistance to address a public health situation, Indigenous Services Canada can draw on these relationships and networks to translate information for action, identify and consult with stakeholders on needs, priorities and perceptions, to monitor the situation, to encourage participation in the response, and to engage communities in creating longer term solutions.
Canada has the expertise and experience to implement social mobilization and social marketing techniques in support of an emergency response when required at all levels. In an emergency, Canadian jurisdictions will tailor their communications strategies to the needs of the event often using more than one approach to achieve their communications goals. Federal, provincial and territorial governments work together to create clear and consistent messaging that local public health networks then use in their tailored communications strategies.
Community listening and feedback
As mentioned, Health Canada and the Public Health Agency of Canada have a toll-free number and public information email address through which members of the public may ask for information on federal services and provide feedback on their health concerns.
Although not a formalized function, the Public Health Network Communications Working Group can and has shared lessons learned with other levels of government. The federal government also participates in the Global Health Security Action Group Communications Working Group, which allows it to reach out to foreign governments to share best practices and lessons learned.
At the provincial and territorial level, liaison between at-risk or affected populations and response agencies is conducted in a systematic fashion. Working relationships are maintained, as appropriate, with response agencies through a variety of working groups, networks and other mechanisms. As noted in the example above for Ontario, many jurisdictions use social media to reach specific communities and to support two-way conversations on public health issues.
If there is evidence of public misinformation, efforts are made to provide up-to-date, accurate information targeted to the affected communities.
R.5.5 Dynamic listening and rumor management
The capacity for listening, detecting and responding to rumours varies across the country. The Government of Canada works diligently to ensure that Canadians always have the most up-to-date and accurate information possible and seeks to counter public misinformation as much as possible. Health Canada and the Public Health Agency of Canada detect and correct rumours and misinformation on a case-by-case basis.
Feedback from the public helps to modify communication strategies and to provide senior management with communications advice to improve responses during emergencies and to counter misinformation as required. The Public Health Agency of Canada and Health Canada have permanent resources dedicated to traditional and social media monitoring that listen for rumours and misinformation regarding public health issues. Regional staff, with close ties to local media and stakeholders, are able to gather information from various sources. Finally, the federal government conducts ongoing public opinion research on a wide range of topics that can identify misinformation and support the development of appropriate messaging to counter it.
The Public Health Agency of Canada gathers and assesses open source information around the world through the Global Public Health Information Network system. The system gathers information from:
- Local, national and international news reports from around the globe in nine languages
- Internet searches of government web sites, ministries of health publications, etc.
- Internet chatting
- Social media networks
- Blogs
- E-mails and messages from partner organizations (WHO, U.S. CDC)
- Health expert reports in various areas, fields and levels
The capacity to respond to rumours, particularly on social media, poses significant challenges in the 24/7 media and social media environment. In an emergency situation where rumours can propagate quickly, resources may be directed towards correcting misinformation and rumours in real time. When the choice is between getting health recommendations out the door and responding to rumours, focus is usually placed on getting factual information out first as opposed to directly responding to misinformation and rumours. The federal government uses a variety of means to address misinformation, including direct responses to media, technical briefings and social media postings. Departments and agencies regularly share messaging with provincial and territorial counterparts to further disseminate factual information based on evidence and current recommendations. Provincial and territorial governments share lessons learned with each other through many formal and informal channels, including the Public Health Network Communications Working Group.
Best practices, challenges, gaps and recommendations
In Canada the field of risk communications is evolving. Current networks, processes and emergency mechanisms have been developed to support an effective response. This effort has helped to develop a culture of sharing expertise and of strengthening working relationships and networks that facilitate coordination and consistent communications during emergencies.
However, the intense demands for communications during a large scale or special hazard event could place a strain on Canada's existing capacity. Ensuring a well-trained, sufficient number of risk communicators to provide surge capacity is a challenge. To help alleviate this challenge at the federal level, departments and agencies have started to work together through the Government of Canada Emergency Communicators Network to enhance information sharing and expertise across the government.
Additionally, efforts are ongoing to promote the incorporation of best practices in risk communications throughout departments and agencies, and not just within communications organizations. This will help to strengthen understanding of risk communication while at the same time better integrating risk communications awareness throughout the multiple levels of government involved in emergency response in Canada.
The Government of Canada will also look to ensure that communications functions can be sustained and avoid personnel "burn out" during long-term or large-scale events. This would support resource sharing in times of emergency where one department or agency could borrow risk communications resources from another as required.
Points of entry
Joint external evaluation target: States Parties should designate and maintain the core capacities at the international airports and seaports (and where justified for public health reasons, a State Party may designate ground crossings) which implement specific public health measures required to manage a variety of public health risks.
Level of capability in Canada
Canada has established an effective system to detect and limit the introduction of communicable diseases into Canada. In 2009, Canada identified, and has since maintained, capabilities at all its IHR-designated points of entry. This is a shared responsibility between the federal, provincial and territorial governments, and industry partners. The Public Health Agency of Canada (PHAC) collaborates with the Canada Border Services Agency and travel and health partners to create a safe public health environment for travellers.
There are more than 140 international airports in Canada (including Canadian Forces airports and water aerodromes) and more than 200 seaports. Five of these are IHR-designated points of entryFootnote 11: two seaports (Port Metro Vancouver and Port of Halifax) and three airports (Vancouver International Airport, Toronto's Pearson International Airport and Montreal's Pierre Elliott Trudeau International Airport). There are also five Quarantine Stations in Canada located in Vancouver, Calgary, Ottawa, Toronto and Montreal.
The Quarantine Act and specific airport or seaport communicable disease response plans help to establish effective public health controls at points of entry. These measures contribute to the protection of travellers and help prevent the introduction of communicable diseases in Canada.
Airline crews, ship crews and Canada Border Services Agency border service officers are all trained to identify travellers who meet syndromic case definitions and to report these individuals immediately. Airports and seaports have agreements with their provincial and local health authorities for the transport, assessment and care of ill travellers.
PHAC is authorized to issue Ship Sanitation Control Certificates and Ship Sanitation Control Exemption Certificates to vessels embarking on international voyages. Airport and seaport sanitation is another key activity that contributes to a safe public health environment. Seaport and airport operators are required to respect sanitation requirements, while also adhering to other provincial and federal requirements.
Indicators
POE.1 Routine capacities are established at points of entry
Identification, quarantine and transfer to medical care at points of entry
PHAC, through its Office of Border and Travel Health, is responsible for the administration of the Quarantine Act (S.C. 2005) at all Canadian international points of entry. PHAC collaborates with the Canada Border Services Agency, airline and ship operators, and provincial and local health partners to provide a safe travel environment and a safe public health environment by identifying and reporting ill travellers.
Quarantine officers are designated by the Minister of Health under the Quarantine Act. All quarantine officers are nurses employed by the federal government who have completed specialized training, which includes both classroom time and on-the-job training.
Quarantine officers work out of quarantine stations in Vancouver, Calgary, Toronto, Ottawa and Montreal, but can also provide services remotely. Accordingly, quarantine officers are available to cover Canada's IHR-designated airports (Vancouver, Toronto, and Montreal) and seaports (Vancouver and Halifax).
Screening and quarantine process
Canada Border Services Agency border services officers are trained and designated under the Quarantine Act as screening officers and are located at all official points of entry to Canada to screen arriving travellers for signs and symptoms of communicable diseases. They are also responsible for implementing regulations related to goods and cargo, as well as administering the provisions of the Immigration and Protection of Refugees Act related to the protection of public health.
To fulfill these responsibilities, border services officers examine baggage, cargo, containers, conveyances, goods, postal parcels and human remains to ensure they are safely handled and free of sources of infection or contamination.
Conveyance operators have a responsibility to report suspected communicable disease cases under Canada's Quarantine Act and the IHR, through the Maritime Declaration of Health (art. 37 and Annex 8) or the Aircraft General Declaration (art. 38 and Annex 9). Conveyance operators must inform the seaport or airport as soon as possible before the vessel arrives at the point of entry if they suspect a person on board meets the syndromic case definition for a communicable disease of concern.
When alerted by the Canada Border Services Agency a conveyance operator, an airport authority or a seaport authority, quarantine officers assess ill travellers at the point of entry, either in person or remotely.
In remote assessment situations a complete health assessment of a traveller (physical assessment and measurement of vital signs) may require support from the local paramedic service.
Provisions of the Quarantine Act grant quarantine officers special authorities to require travellers to undergo a medical examination or report to a medical facility for further diagnosis and treatment when there is a suspected communicable disease of public health concern. At airports with quarantine stations, designated assessment and isolation areas are available where travellers can be screened or held before they are transferred to the local health authority.
Provinces and territories are responsible for the management and transportation of ill travellers from the point of entry to a hospital for diagnosis and treatment. Local paramedic services transfer patients to the hospital following provincial and territorial standard operating procedures, which are based on the principles of good patient care, such as those laid out in Ontario's Patient Care and Transportation Standards from Ontario's Ministry of Health and Long Term Care. (See section R2: Emergency response operations.)
Figure 7: IHR designated points of entry in Canada - Text description
A map of Canada is used to identify the IHR Designated Points of Entry in Canada, of which there are five – three airports and two seaports. The map uses a white star to identify the national capitol, and white asterisks to identify provincial/territorial capitals. Large yellow stars highlight the areas within provinces that have designated points of entry (British Columbia, Ontario, Quebec and Nova Scotia). In British Columbia, an airplane icon and a ship icon are used to highlight the Vancouver International Airport and The Metro Vancouver Cruise Ship Terminal, respectively. In Ontario, Toronto Pearson International Airport is identified with an airplane icon as a designated Point of Entry. In Quebec, an airplane icon highlights Montreal Trudeau International Airport. In Nova Scotia, a ship icon identifies the Halifax Cruise Ship Terminal as the fifth and final designated Point of Entry.
Point of entry inspections and public health safety
Provinces and territories are responsible for ensuring a safe environment in point of entry facilities in their jurisdiction. In most provinces and territories oversight of provincial public health acts and regulations is the responsibility of local municipal public health authorities.
Ontario's Health Protection and Promotion Act and regulations, for example, include provisions on the organization and delivery of public health programs and services; the prevention of the spread of disease; and the promotion and protection of the health of the people of Ontario. This includes the control of vectors and reservoirs, notably with the public health inspection programs for food establishments located at airports and seaports. In addition to these inspections, occupational health safety standards are implemented through provincial and territorial legislation.
At the federal level, PHAC has designated environmental health officers who conduct inspections of conveyances and ancillary services under the following acts and regulations:
- Quarantine Act
- Food and Drugs Act
- Department of Health Act
- Potable Water on Board Trains, Vessels, Aircraft and Buses Regulations
- International Health Regulations
Environmental health officers have expertise in food, water and general sanitation. All environmental health officers have national certification as public health inspectors in Canada and are trained and designated under the Quarantine Act as screening officers.
Environmental health officers are based out of offices located across Canada-in Vancouver, Toronto, Montreal, Quebec City, Halifax, Moncton, and St. John's-and provide services to Canada's IHR-designated seaports and airports, as well as to Canada's 200 authorized seaports.Footnote 12 Services provided by environmental health officers include:
- Public health inspections, support for development of industry management systems
- Technical guidance for targeted conveyance sectors
- Outreach and food handler training for industry
- Complaint and outbreak investigation
Services target passenger conveyances (trains, airplanes, cruise ships, ferries, buses) and ancillary services that support them (airport water haulers, flight kitchens, supply depots).
To carry out Canada's obligations under the IHR, PHAC issues Ship Sanitation Control Certificates and Ship Sanitation Control Exemption Certificates to vessels embarking on international voyages that request one. Canada's Department of National Defence is authorized to issue both certificates for the Royal Canadian Navy Department of National Defence vessels (See section P1: National legislation, policy and financing).
POE.2 Effective public health response at points of entry
Support for public health emergency response at points of entry
Since all three levels of government (municipal, provincial/territorial, federal) are represented at most points of entry, there is a need for integration and cooperation. Canada's suite of emergency response plans guide and coordinate activities; those plans that come into play at points of entry include:
- Federal Emergency Response Plan
- Health Portfolio Emergency Response Plan
- Federal, Provincial, and Territorial Public Health Response Plan for Biological Events
Each IHR designated point of entry has a communicable disease response plan as an annex to the airport or seaport emergency response plan. Recognizing that response to a communicable disease at a point of entry is a shared responsibility, these plans detail the roles of the various agencies and how they engage with each other. The agencies identified in the communicable disease response annex are consulted in the development of the plan. PHAC is currently conducting an environment scan of these communicable disease response plans for points of entry in order to identify best practices and develop a guidance tool that will be shared with all airports and seaports for consideration when revising or updating their response plans. Response plans are tested through exercises on an annual basis.
British Columbia and Nova Scotia also have Integrated Health Response Plans for migrant vessels to address how health partners from local, provincial, and federal organizations prepare for and respond to the arrival of a migrant vessel in their province.
The Canada Border Services Agency has policy, guidelines and procedures that also support the delivery of services at the point of entry, such as the Pandemic Guide and the Emergency Preparedness All Hazards Approach Manual.
An umbrella memorandum of understanding defines the basis for ongoing cooperation and coordination between the Canada Border Services Agency, Health Canada, and PHAC in the administration and enforcement of acts and regulations covering travelers, conveyances, and cargo.
International capacity building
Canada is considered a leader in the global community in the area of ship sanitation inspections. PHAC works with the Pan American Health Organization and World Health Organization on international capacity building efforts by offering training and guidance on conveyance inspections. While not officially published, this expertise is recognized and has been shared with others upon request.
Best practices, challenges, gaps and recommendations
Canada's public health capacity at designated points of entry is well established and PHAC quarantine stations are well supported through collaborative efforts with other point of entry partners. The collaboration between PHAC quarantine officers and the Canada Border Services Agency border service officers in particular ensures that public health measures are in place at all official points of entry in Canada. Canada's integrated response at points of entry is based on coordination and collaboration between all partners-from airline and ship operators to local public health authorities.
Points of entry communicable disease response plans are owned and maintained by individual airports and seaports. While these plans are, in most cases sufficient, PHAC would like to see more consistency among these plans, which is why a national guidance document is currently being developed.
PHAC is in the midst of a modernization initiative, driven by lessons learned from public health events, such as the Ebola outbreak in 2014-16, as well as by recommendations from previous audits and evaluations. This modernization initiative will ensure Canada's program areas are well suited to meet current public health challenges, including those posed by increased global travel, tourism and trade.
Chemical events
Joint external evaluation target: States Parties should have surveillance and response capacity for chemical risk or events. It requires effective communication and collaboration among the sectors responsible for chemical safety, industries, transportation and safe disposal.
Level of capability in Canada
Canada is signatory to the International Health Regulations (IHR 2005). Its scope and purpose are to prevent, protect against, control and provide a public health response to the international spread of disease in ways that are commensurate with and restricted to public health risks, and which avoid unnecessary interference with international traffic and trade. For the purposes of the IHR, disease means an illness or medical condition, irrespective of origin or source, including chemical events, that presents or could present significant harm to humans.
The IHR includes an algorithm with four criteria for the assessment and the notification of events that may constitute a public health emergency of international concern. The scope of the technical section on chemical eventsFootnote 13 covers more broadly those capabilities and the enabling environment available in Canada to respond to those events regardless of its cause, location, or magnitude.
The manufacturing, storage, transport, use and waste-disposal of chemicals world-wide pose a risk of accidental or intentional release of hazardous chemicals, which can impact public health. Chemical events can result from human activities such as industrial leaks at storage facilities or from transportation of dangerous goods, fire and explosions, food and consumer product contamination. Natural sources, such as volcanic eruptions or forest fire are also of concern.
All the above scenarios tend to be accidental. The current global situation sets the tone for deliberate chemical releases using more readily available toxic industrial chemicals (i.e. the hijacking of a chlorine tanker during transport) or the potential release of chemical warfare agents which is more complex to achieve, although possible, as demonstrated in the recent events reported in Syria-Iraq war conflict area.
Canada maintains a significant level of preparedness and response capability for chemical events. This capacity is multi-layered between the various levels of government and industry with mechanisms and collaboration agreements in place to support the response. The magnitude of the response is scalable, depending on the severity of the event.
Canada also has an extensive regulated environment for the management of chemicals and is signatory to a number of international treaties, conventions, response protocols and non-proliferation agreements that further contribute to the capability.
The level of response capabilities for chemical events varies somewhat across Canada. This partly depends on the geographic area; urban areas generally have more capability than rural areas. It also depends on the level of chemical manufacturing activity in a given area. Typically, the greater the levels of industrial chemical activity present in one area, the more capabilities are available. Finally, it also depends on the size of jurisdictions. Larger governments tend to have greater capabilities resulting from the amount of dedicated resources. Despite these differences, certain commonalities exist which are described below.
Local capabilities (at the site of a chemical event)
Most chemical emergencies (events) are localized and managed by resources at the site. There are two components to the management of a chemical event; the mitigation of the chemical released and the management of the public health consequences. The industry plays a greater role in the first component whereas first responders and local or regional public health authorities are mostly involved in the second component.
First responders consist of fire (hazmat teams, with variable operational capabilities across the country; mostly concentrated in major urban centres), police and emergency medical services. Most local responders across Canada use some form of Incident Command System to control and coordinate response activities. Imbedded in the Incident Command System is a health and safety function generally assumed by specialists from the municipality and the private sector supported by specialized personnel from provincial and territorial health authorities.
The health and safety function provides public health guidance and coordination of response measures to help manage the health consequences of chemical events. Local hospitals provide medical care for victims of chemical events. The response to chemical events can represent a challenge to hospital staff due to the limited experience or training they receive for such occurrences or the unfamiliarity with certain chemical products.
In many provinces and territories local or regional public health have legislative reporting requirements for health hazards, which implicate them in chemical events.
Industrial (private sector) capabilities
When a chemical event originates from an industrial source (i.e. chemical production, transport, etc.) then industry has obligations, under various regulations, to provide chemical specific resources and expertise to mitigate the chemical event (procedure to stop the leak or to recover spilled chemicals). These capabilities may be provided by response teams from chemical producers, the transportation of dangerous goods industry (e.g. rail) or contractors specializing in emergency response and the disposal of hazardous materials. Transport Canada, through its Emergency Response Assistance Plan verification and approval process, has assessed many of these contractors.
Provincial and territorial capabilities
Provincial and territorial governments become involved when local resources are exhausted or specialized expertise not available at the local level is needed. These capabilities include medical care, poison control and medical toxicology advice, evaluation and management of environmental and public health consequences including psychosocial impacts.
Federal capabilities
The federal government provides support when requested by a province, territory, or an international counterpart or when an emergency occurs in a federal jurisdiction or is cross-jurisdictional. The level of capability consists of further specialized scientific support (e.g. hazard assessment, air/water dispersion modeling, specialized laboratory support and specialized expertise for chemical warfare agents, toxic industrial chemicals, pesticides, drugs, etc.), and medical countermeasures from the National Emergency Strategic Stockpile. In the event of a suspicious attack involving chemicals, additional capabilities would be provided by the National Chemical, Biological, Radiological, Nuclear and Explosives (CBRNE) Response Team led by the Royal Canadian Mounted Police.
Indicators
CE.1 Mechanisms are established and functioning for detecting and responding to chemical events or emergencies
Incident detection
Canada has mechanisms in place to detect and respond to chemical events. These mechanisms rely on a comprehensive regulatory environment and on networks of experts sharing knowledge and specific laboratory capabilities. There are several mechanisms to detect chemical events which are used regardless of the magnitude and the nature of the event-whether accidental or deliberate.
Direct notification
Detection can occur locally when an individual observes a chemical event (spill, explosion, fire, etc.). First responders can also detect a chemical event when they conduct routine inspections planned as part of prevention activities (firefighters) or when responding to an emergency dispatched by the emergency call centre (911).
Trends from various data sources
Health care professionals in hospitals may detect an abnormal situation when they receive an unusual number of patients with similar symptoms. Canadian poison centres are an invaluable source for detecting new poisoning sources or trends and informing public health authorities by answering calls from the public and the health care professionals.
At the provincial and territorial level, there is an array of data sources to conduct detection and surveillance activities for chemical events. This includes coroners' or medical examiners' data, poison centres data, public health, telehealth data, and mandatory reporting for specific chemical exposures (in some jurisdictions).
Sample analysis
Highly specialized toxicology laboratories are useful to analyze biological specimens and environmental samples to identify and confirm the nature of the chemical released and inform response and consequence management. Such specialized laboratories are mainly available at the provincial, territorial and federal level as well as in a few major cities across Canada.
Inspection activities and reporting enabled by regulatory requirements
At the federal level, there are obligations to report the release, anticipated release, loss, or theft of specified chemicals above certain thresholds. For example, the Transportation of Dangerous Goods Act requires an immediate notification to the local authorities responsible for the emergency response when a spill occurs.
The Canadian Environmental Protection Act, 1999 and the Fisheries Act, require those responsible for an emergency to notify the government of a real or potential pollution release and to take appropriate response measures. A streamlined federal/provincial/territorial environmental notification system uses 24-hour authorities as the first point of contact. The system tracks and transfers information between authorities for timely and effective oversight of the response.
Within the Health Portfolio, there are regulatory programs that conduct inspections on different types of consumer products, medical devices, foods, drugs and natural products as well as drinking water in First Nations communities. These programs conduct routine inspections as part of the regulatory compliance process or when an abnormal situation is reported by consumers or medical professionals, such as by hospital or poison centre staff. These inspections are supported by federal laboratory capabilities.
The Canadian Food Inspection Agency surveillance and inspection programs are designed to detect the presence of hazards in food, animals and plants and their products, and provide an early warning for problems whether they are accidental or intentional.
Canada demonstrates capacity to exchange information between appropriate chemical units at the different government levels for detection purposes. For example, the monitoring of different matrices such as air and water could lead to the detection of a chemical contamination. This activity is part of the day-to-day operations of the regulatory authorities at the various levels of government. Both provincial and federal government have capabilities to conduct such monitoring. In Canada, the responsibility for ensuring drinking water supplies are safe is shared between the federal, provincial, territorial, and municipal governments.
International treaties, protocols and standards
Canada is signatory to the following treaties:
- Rotterdam Convention on the Prior Informed Consent Procedure for Certain Hazardous Chemicals and Pesticides in International Trade
- Stockholm Convention
- Basel Convention
- Strategic Approach to International Chemicals Management
- United Nations Economic Commission for Europe on the Transboundary Effect of Industrial Accidents
These treaties aim to enhance environmental public health safety in Canada by promoting:
- Efficient methods for exchanging information about industrial chemicals and pesticides to implement law enforcement mechanisms and concerns about specific chemicals such as the persistent organic pollutants
- Regulatory measures based on strong science, assessment, management and monitoring tools
- International controls for transportation and waste disposal and preventing industrial accidents
- Global chemical safety resulting from the identification of emerging issues
National chemical response plans
Canada has an extensive suite of policies (e.g. acts, regulations, plans) that address chemical events. Both federal and provincial/territorial jurisdictions are involved to ensure national oversight.
As referenced in section R1: Preparedness, the Emergency Management Act (2007), section 6(3) states that Government of Canada institutions may not respond to a provincial emergency unless the government of the province requests assistance or there is an agreement with the province that requires or permits the assistance. However, in extreme circumstances, the Emergencies Act (1985) provides the Government of Canada the authority to take special temporary measures to ensure safety and security during national emergencies. This includes chemical emergencies that seriously endanger the lives, health or safety of Canadians and are of such proportions or nature as to exceed the capacity or authority of a province to deal with it, or seriously threaten the ability of the Government of Canada to preserve the sovereignty, security and territorial integrity of Canada.
The Emergency Management Act, (2007) sets out clear roles and responsibilities for federal ministers across the full spectrum of emergency management, including prevention/mitigation, preparedness, response and recovery. In preparation for emergencies, federal departments work in close partnership with other levels of government, industry, and communities to identify potential risks, to develop and exercise contingency plans and to train personnel.
The Emergency Management Act (2007), section 6(1), requires each minister accountable to Parliament for a government institution to identify the risks that are within, or related to, that minister's area of responsibility and to:
- Prepare emergency management and response plans in respect of those risks
- Maintain, test and implement those plans
- Conduct exercises and training in relation to those plans. (Health Portfolio Policy for Chemical Emergency Management)
Under the Emergency Management Act (2007), the Minister of Public Safety is responsible for coordinating the Government of Canada's response to an emergency. The Federal Emergency Response Plan is the Government of Canada's "all hazards" response plan. The Plan identifies the emergency support functions providing the mechanisms for grouping certain functions. Specifically, these are the functions most frequently used in providing federal support to provinces and territories or assistance from one federal government institution to another during an emergency.
The Federal Emergency Response Plan establishes the Health Portfolio, which comprises Health Canada and the Public Health Agency of Canada as the primary departments for Emergency Support Function #5: Public Health and Essential Human Services. It specifies that the Health Portfolio will provide technical advice and support for chemical emergencies.
For Emergency Support Function #6: Environment, the Health Portfolio provides support to Environment and Climate Change Canada to mitigate public health impacts related to an environmental emergency. The Health Portfolio Emergency Response Plan was developed to fulfill this role.
There are memoranda of understanding such as The Operational Framework for Mutual Aid Requests that may be of assistance during Health Portfolio emergency response activities. The Operational Framework is a non-binding mechanism that can be activated by provinces or territories to identify and share healthcare professionals and health assets inter-jurisdictionally during events. Although originally created for physicians and nurses, the tool is flexible and can expand to identify other healthcare professionals, such as environmental health officers. It aims to streamline the mutual aid request and offer processes. The Health Portfolio Chemical Emergency Response Annex was developed and is regularly updated as an operational complement to the Health Portfolio Emergency Response Plan.
Expertise and resources within the Health Portfolio for response to chemical emergencies are located in more than 20 different programs.
The Health Portfolio's key partners for chemical emergency management are:
- Provincial and territorial health authorities and emergency management offices
- Federal departments, including Public Safety Canada, Transport Canada's Canadian Transport Emergency Centre (CANUTEC), National Defence's Defence Research and Development Canada, Environment and Climate Change Canada's National Environmental Emergency Centre, Canadian Food Inspection Agency, Global Affairs Canada, and the scientific network via the Centre for Security Science.
- International: Member countries and organizations of the Global Health Security Initiative and the World Health Organization.
The Government Operations Centre, administered by Public Safety Canada, provides an all hazards integrated federal emergency response to events (potential or actual, natural or human-induced, accidental or intentional) of national interest. It provides 24/7 monitoring and reporting, national-level situational awareness, warning products and integrated risk assessments, as well as national planning and whole-of-government response management. During periods of heightened response, the Government Operations Centre is augmented by staff from other government departments and agencies, as well as non-governmental organizations whose representatives physically work in the Government Operations Centre and connect to it virtually.
The Government Operations Centre ensures national coordination of chemical events with numerous federal departments and agencies via their respective emergency coordination centres. The Health Portfolio Operations Centre liaises simultaneously with multiple health stakeholders at different government levels such as Health Canada's Chemical Emergency Preparedness and Response Unit, which coordinates the chemical expertise within the Health Portfolio programs and with provincial and territorial public health departments and agencies.
Other federal departments are involved individually or collectively in the management of chemical events given their mandates as described below.
Transport Canada develops safety and security regulations, means of containment standards, provides oversight and expert advice on dangerous goods safety and security incidents to promote public safety in the transportation of dangerous goods by all modes of transport in Canada.
Environment and Climate Change Canada protects Canadians and their environment from the effects of environmental emergencies through the provision of science-based expert advice and regulations.
Canadian Food Inspection Agency is an integral part of the federal government's capacity to respond rapidly and effectively in the event of a food safety emergency or a threat to agricultural or forest biosecurity.
The Royal Canadian Mounted Police coordinate Canada's National Chemical, Biological, Radiological, Nuclear and Explosives Response Team. Composed of experts from several departments, the team evaluates scenes potentially linked to terrorist or suspected terrorist activity. Such events typically involve the use of devices that can cause damage or injury over a large area, for example poisonous gases or explosives. The team looks for trace evidence and collects samples to determine if a chemical, biological radiological or nuclear weapon of mass destruction was used.
The CBRNE Team can also be deployed to major events, such as the Olympics or the G8 and G20 summits, to provide an immediate on-site analysis of suspicious packages, attacks or unexpected incidents. When requested, the CBRNE team provides assistance to police agencies following a CBRNE event and is available to provide police agencies with CBRNE training.
The Canadian Coast Guard is the operational arm of the Government of Canada responsible for ensuring an appropriate response to ship-source and mystery-source pollution incidents in Canadian waters. This constitutes a major component of the overall marine pollution response capacity in Canada. The Environmental Response Program of the Canadian Coast Guard is responsible for preparedness and response in this regard. The Environmental Response Marine Spills Contingency Plan defines the scope and framework within which the Canadian Coast Guard will operate to ensure a response to marine pollution incidents. In accordance with Canada's Marine Oil Spill Preparedness and Response Regime, the polluter is expected to respond to incidents while the Canadian Coast Guard will monitor and, whenever necessary, augment or assume management of the response when it is in the interest of the public. The Canadian Coast Guard may also provide assistance to other federal, provincial, territorial or local agencies.
This suite of policies illustrate that Canada has response plans that define the roles and responsibilities of relevant agencies at the national level. The phases of the emergency management cycle are recognized in these acts, regulations and plans. However, it is recognized at different government level that gaps to the recovery phase currently exist.
In general, the roles and responsibilities for response to accidental events are better understood and coordinated by responders at all levels of government and the private sector. This is based on the fact that such responses are routinely done and exercised. Conversely, Canada has largely been spared from intentional events. Despite response planning and preparedness at various government levels, the lack of practical experience in responding to intentional events has limited the confidence and knowledge-building of responders with respect to their roles and responsibilities. Continuous improvement in the response to accidental events will also improve the ability to respond to intentional events.
Risk assessment and exposure monitoring
Population health risk assessment is the product of hazard assessment and human exposure assessment. While the data on chemical hazards might be available from scientific literature, the federal health authorities can support the hazard assessment when data on chemical hazards is scarce or limited (e.g., in the case of a new chemical).
Data exposure collection is done by various jurisdictions in Canada; data on human exposure is mainly collected and assessed by provincial and territorial governments as required. Some of the larger cities have the capacity to collect data following a chemical event. When requested, support from the federal level can be provided to the provincial and territorial governments in determining the appropriate public health response to implement, for instance, by comparing exposure data with exposure guideline values (i.e. for air and water quality). As a complement, the federal level can support environmental sampling and epidemiological investigations, recognizing that the latter is not systematically performed after each chemical event.
Environmental monitoring
There are two different types of analysis to estimate human exposure from environmental sampling:
- At the site, with field instruments for rapid qualitative presumptive analysis
- In a laboratory with instruments of greater accuracy for quantitative analysis
These are undertaken by the department of the environment at the local or provincial level, when required. They are done at the federal level, occasionally, if within a federal jurisdiction or upon request from a province or territory.
At the provincial and territorial level, one example of field detection equipment for environmental sampling is the Trace Atmospheric Gas Analyser (Available in French only) mobile laboratories. These are available to support emergency response measures in certain provinces. They monitor air quality and assess contaminants released into the air. The Trace Atmospheric Gas Analyser helps to calculate the concentration of contaminants in the air to determine the area affected following a chemical release. Therefore, decision makers are provided with a rapid assessment of the situation.
At the federal level, one example of a laboratory network that can support provincial and territorial environmental sampling is the Health Portfolio Chemical Laboratory Network, which includes the following seven Health Canada members:
- Environmental Health Science and Research Bureau, Environmental and Radiation Health Sciences Directorate
- Product Safety Laboratory, Product Safety Program
- Bureau of Chemical Safety, Health Products and Food Branch
- Laboratory Services, Pest Management Regulatory Agency
- Regional Food Laboratories, Regulatory Operations and Regions Branch
- Inspectorate Laboratory Programme, Regulatory Operations and Regions Branch
- Drug Analysis Service, Regulatory Operations and Regions Branch
Human health monitoring and biospecimen analysis
Canada's five poison centres collect exposure data as part of their regular operations. The data can support health authorities by providing valuable information to support risk assessment and management at all levels of government. However, the data is not currently aggregated and analyzed at the national level.
Human exposure can also be measured directly from clinical samples (e.g. blood, urine). This is predominantly done in hospital laboratories or specialized clinical toxicology laboratories at the provincial level. Health Canada has recently created an emerging network, supported by the Canadian Network for Public Health Intelligence, of such specialized clinical toxicology laboratories (i.e. Toxicology Laboratory Response Network) to act as surge capacity and analytical information sharing for response to chemical events. Additional tiered surge capacity can also be provided for clinical samples by specific laboratories of the Health Portfolio Chemical Laboratory Network.
Epidemiological investigations
Epidemiological investigations can be conducted by local and provincial and territorial government authorities and, when required federally, by the Public Health Agency of Canada's Canadian Field Epidemiology Program, which builds Canada's public health capacity by mobilizing field epidemiologists anywhere they are needed within Canada or around the world, supporting public health organizations as they respond to urgent public health events.
Given that follow-up or studies on population health are not conducted systematically after each chemical event in Canada, there is no standardized, ready-to-use tool at the federal level similar to the Community Assessment for Public Health Emergency Response a U.S. tool designed to provide timely and low-cost household-based information about a community.
Mitigation and treatment
In Canada, mitigation of a chemical event is done most often at the local level, with initial response done by local first responders, and escalated to provincial, territorial and federal levels only when necessary. Industry provides response expertise as many mutual aid agreements are in place. Local capabilities vary across the provinces and territories as hazmat teams are not present in every region and have different detection equipment to initiate the response to a chemical event.
At the provincial level, the ministry of the environment is most often the first line of support to front line responders. When requested, the federal level has several response programs, including response teams in such federal departments as the Canadian Coast Guard, Environment and Climate Change Canada, the Health Portfolio and Transport Canada.
Environment and Climate Change Canada's National Environmental Emergencies Centre key role is to provide the Department's technical and scientific environmental advice and assistance to better prevent, prepare for, respond to and recover from environmental emergency. Available 24/7, the National Environmental Emergencies Centre offers a number of services to effectively manage an environmental emergency and reduce its impacts, such as:
- Site-specific weather forecasting (provided by Environment and Climate Change Canada's meteorological services)
- Spill trajectory modelling
- Fate and behaviour of hazardous substances
- Environmental sensitivity mapping
- Establishment of clean-up priorities
The Health Portfolio focal point for the management of chemical emergencies is Health Canada's Chemical Emergency Preparedness and Response Unit, which is responsible for 24/7 situational awareness, initial assessment and notification of chemical emergencies to concerned Health Portfolio programs, and for the coordination of scientific expertise for response to chemical emergencies. The Duty Officer has 24/7 access to a comprehensive list of chemical databases to support the Health Portfolio's response to chemical events. Most responders from the chemical emergency response community use well-known tools, such as the Agency for Toxic Substances and Diseases Registry (TOXPROFILES), RightAnswer, Chemical Hazards Emergency Medical Management, 2016 Emergency Response Guidebook, ChemPendium, and the Emergency Response Decision Support System.
Transport Canada's Canadian Transport Emergency Centre is a national advisory service available to assist emergency response personnel in handling dangerous goods emergencies on a 24/7 basis. The Centre's staff includes bilingual scientists with specialization in chemistry or a related field and training in emergency response. Emergency response advisors are able to interpret technical information from a variety of scientific sources.
Guidance to respond to chemical events is available in the Emergency Response Guidebook (2016). Transport Canada remedial measures specialists are also available during emergencies to provide advice on transportation of dangerous goods emergency response procedures. They also attend at dangerous goods transportation emergencies when required.
Dangerous goods that require special expertise and equipment in response to an incident must have an emergency response assistance plan during transport. These plans provide local emergency responders with trained and equipped emergency response personnel and technical experts at the scene of the incident. They also provide a detailed picture of the custodian's capability to respond to a chemical event.
Initial life-saving care for victims of a chemical event is provided by local emergency medical services. Once stabilized, patients are transported to hospitals where medical professionals provide treatment.
Specialized clinical toxicology expertise, such as guidance for the administration of chemical antidotes, can be provided by the five poison centres in Canada. Most provinces and territories that do not have a poison centre have some type of agreement with a neighbouring provincial poison centre for service coverage. Poison centres are managed by the provinces:
- Drug and Poison Information Centre, for British Columbia and the Yukon
- Centre antipoison du Quebec, for Quebec
- IWK Regional Poison Centre, for Nova Scotia and Prince Edward Island
- Ontario Poison Centre, for Ontario, Manitoba and Nunavut
- Poison and Drug Information Centre, for Alberta, Saskatchewan and the North West Territories
The poison centres rely on the expertise of medical toxicologists for specific advice to treat patients severely exposed to chemicals, especially when the administration of a chemical antidote is part of the treatment. Chemical antidotes are available at the local, provincial and federal levels. The stockpiles are variable across Canada as they are most often managed by the local health facilities (i.e. hospital pharmacies) in collaboration with the provincial health authorities.
The provincial stockpiles are not located physically in the poison centres as it is beneficial that chemical antidotes be available at the local level for rapid administration supported by advice from medical toxicologists, as required. At the federal level, the National Emergency Strategic Stockpile stockpiles some medical countermeasures for specific chemical threats. Federal assets include equipment and medication. The deployment of National Emergency Strategic Stockpile assets is done when requested by a province or a territory in response to a chemical event and occasionally in anticipation of a mass gathering event (e.g. the Olympics).
Under current consideration are information-sharing mechanisms and tools, such as an antidote registry, to obtain a detailed picture of chemical antidote stockpiles available across the country.
The Health Portfolio in collaboration with federal, provincial and territorial health authorities, the provincial poison centres, non-governmental organizations and international contributors-is developing a Canadian Surveillance System for Poison Information that can aggregate, analyze and interpret poison centre data to provide timely surveillance and national statistics on poisonings. It will be an application on the Canadian Network for Public Health Intelligence that will provide near-real-time capability to collate and visualize the data, as well as identify potential safety signals.
Canada has demonstrated response capacity because of the multiple plans that are in place. There is an existing notification system that would benefit from improved communication between the health and environmental agencies to enhance information sharing for more timely and efficient response.
Canada's surveillance capacities will be increased once data collected by the poison centres are aggregated at the national level. There is a lot of exercising and technical capacity to detect chemicals, even though challenges remain to ensure adequate information sharing about detection capabilities (expertise and equipment) across the different government levels. Canada, similarly to other countries, faces the challenge of accessing timely and reliable information about the health and environmental risks of new chemicals entering its market.
CE.2 Enabling environment is in place for management of chemical events emergencies
Chemical safety and hazard regulations
Canada's strategies and processes are designed for prevention and mitigation, preparedness, response, and recovery from chemical emergencies where every order of government (municipal, provincial, territorial, federal) plays a part in protecting against risks from chemical substances.
Strong science, assessment and monitoring, combined with a variety of tools for protection: this is a risk-based approach to chemical substances in Canada. The Government of Canada makes regulations and develops guidelines and objectives that apply across the whole country. It also leads in conducting scientific research on human health and environmental issues, and is signatory to agreements with other countries to ensure chemical safety. The aim is to safeguard human health and the environment in a global context while supporting economic growth. This is the essence of sustainable development.
Emergency management in Canada is a shared responsibility, which relies on ongoing cooperation and communication between all levels of government. Within Canada's constitutional framework, the provincial and territorial governments and local authorities provide the first response to the majority of emergencies.
The National Emergency Response System provides for the harmonization of joint federal, provincial and territorial response to emergencies. It supports and facilitates procurement and logistics coordination between all levels of government, the private sector, non-governmental organizations and international stakeholders. Although in most instances it applies to federal support at the request of a province or territory, it can also be used in instances where provinces or territories support federal response to an emergency under federal jurisdiction.
Each province and territory has emergency management legislation. Generally, these acts and regulations set out the common roles of the provincial and territorial ministers, and municipalities that are responsible for dealing with emergency management in each jurisdiction, and specify the extraordinary powers and declarations of emergencies that may be implemented. Provincial and territorial legislation also identifies the extraordinary powers that provincial and territorial authorities may use and the circumstances and safeguards under which those powers may be exercised.
Each province and territory has developed its own governance structure for coordinating the response to emergencies within its jurisdiction. While each province or territory has customized its governance to suit its unique and specific requirements (geographical, cultural, etc.), most have broadly similar organizational structures with a significant degree of commonality among their mechanisms and procedures.
Provinces and territories have strategic, operational and tactical responsibilities similar to the federal government with respect to the management of emergency response that occur within their jurisdiction.
At the federal level, within the mandate of several departments, health and environment are protected through numerous laws that govern chemical substances, including those in food, drugs, pesticides and consumer products. There are also laws covering the release of pollution into air, water, and natural wildlife habitats.
The federal government is responsible for over 25 different laws covering environment and environmental health issues in addition to laws to address potential emergency situations accidental or deliberate in nature. The key Canadian regulations related to chemical safety and emergency response that provide the legislative framework for managing chemicals are:
- Canadian Environmental Protection Act (1999) and Regulations Designating Regulatory Provisions for Purposes of Enforcement
- Emergency Management Act (2007) and regulations
- Transportation of Dangerous Goods Act and Transportation of Dangerous Goods Regulations
- Hazardous Products Act and regulations
- Consumer Product Safety Act and regulations
- Pest Control Products Act and regulations
- Food and Drugs Act and Food and Drug Regulations
- Fisheries Act and regulations
- Canada Labour Code and regulations
- Canada Shipping Act and regulations
Provincial and territorial governments and municipal authorities also have regulations aiming to protect health and environment in their jurisdictions and mandates. The Canadian regulatory environment enables a safe environment for all Canadians.
Canada has also made international commitments to enhance global health security by its participation in the Global Health Security Initiative and by adhering to the IHR. Health Canada works closely with Canada's IHR National Focal Point on all international reporting requirements to the WHO for chemical events.
Canada is part of bilateral agreements with the United States to respond to chemical events with potential impacts on both sides of the border, such as the Canada-United States Joint Marine Pollution Contingency Plan for Spills of Oil and Other Noxious Substances. It covers the response to pollution incidents affecting or threatening the waters or coastal areas of both parties. The Canadian Coast Guard is the lead agency for Canada.
Similarly, Environment and Climate Change Canada leads, with the U.S. Environmental Protection Agency, the Canada-United States Joint Inland Pollution Contingency Plan (2009). The Plan provides a mechanism for preparedness and response to pollution incidents within 25 km on either side of the inland boundary and facilitates the provision of assistance, when one country is affected and the incident is of such magnitude as to justify a request for assistance from the other country.
Public Safety Canada is the lead on the Chemical, Biological, Radiological, Nuclear and Explosives Resilience Strategy for Canada. In order to enhance and sustain Canada's resilience to CBRNE events, all levels of government within Canada have collaborated to develop a Chemical, Biological, Radiological, Nuclear and Explosives Resilience Strategy for Canada. Its purpose is to provide the policy framework that will guide the creation of sustainable capabilities and common standards in CBRNE policies, programs, equipment and training.
Canada is bound by the following international treaties for marine and aviation transport:
- International Convention for the Prevention of Pollution from Ships
- International Convention for the Safety of Life as Sea
- Convention on International Civil Aviation (also known as Chicago Convention)
International transport of dangerous goods by marine and air modes must be in accordance with the International Maritime Dangerous Goods Code and the Technical Instructions for the Safe Transport of Dangerous Goods by Air, respectively.
Canadian laws support the principle of polluter responsibility, which means industry is accountable for taking adequate preventive actions and for having effective response plans in place. For example, under the Transportation of Dangerous Goods Act, when a shipper transports dangerous goods that require an emergency response assistance plan, the plan must be approved by Transport Canada prior to the shipment taking place. The Environmental Emergency Regulations, under the Canadian Environmental Protection Act (1999), require facilities that manufacture, store, use or dispose of toxic or other hazardous materials in quantities beyond specified thresholds to prepare and implement environmental emergency plans.
Internationally, Canada works with several partners and in multi-lateral fora to advance and share knowledge in the area of emergency prevention, preparedness and response. Canada maintains a significant working relationship with organizations such as the Joint United Nations Environment Programme/Office for the Coordination of Humanitarian Affairs, Environment Unit.
Within the International Maritime Organization's Convention on Oil Pollution Preparedness, Response and Cooperation and the International Convention for the Prevention of Pollution from Ships, Canada has implemented annexes I, II and III which set carriage rules for oil, noxious liquid substances and packaged dangerous goods; this includes hull design, discharge controls, designs for transfer conduits and connections, and operational procedures to promote safety and prevent pollution from spills and accidents.
Canada is also an active member of the Arctic Council, a high-level forum for cooperation regarding the prevention, preparedness and response to environmental emergencies in the Arctic that are a result of human activities or natural disasters.
Canada's Food and Consumer Safety Action Plan proposes measures to support better identification of risks in the food supply, the establishment of preventative risk mitigation approaches, and targeted oversight to verify that industry's preventative approaches are effective and that there is a rapid response when problems do occur. Canada has a long history of cooperation regarding food safety with international regulatory counterparts to leverage resources and knowledge and to apply sound regulatory practices and standards that are consistent with international norms.
Canada collaborates and coordinates many of its risk management efforts with key food regulatory partners in the United States, Europe, Australia, New Zealand, and Japan. Engagements range from informal information exchanges to multilateral harmonization initiatives through international organisations such as Food and Agriculture Organization of the United Nations, Codex Alimentarius Commission, and the World Health Organization.
Currently being implemented in Canada, the Globally Harmonized System of Classification and Labelling of Chemicals is an internationally consistent approach to classifying chemicals and communicating hazard information through labels and safety data sheets. Its key objectives are:
- To increase worker protections through the adoption of an improved, globally recognized standard for communicating the hazards associated with workplace hazardous chemicals
- To facilitate trade through common labelling and other hazard communication requirements
- To lower costs for businesses and consumers by reducing the need for retesting and reclassifying workplace hazardous chemicals from, or for, different markets
Exercises, simulations, and real-world responses
Exercises are regularly done to ensure responders at all levels are prepared to respond to chemical events; from first responders and first receivers, at the local level, to provincial, territorial and federal government departments and agencies with a mandate for the management of chemical events. These exercises can also include industry as it has specialized expertise and significant resources to respond to chemical events.
The Public Safety National Exercise Program serves as the principal mechanism for examining the preparedness and confirming the capability of the Government of Canada to successfully respond to events of national interest. The program is intended to incorporate participants and exercises from a whole-of-government perspective, including partnering with provinces, territories, municipalities and Indigenous communities. When appropriate, international partners will be incorporated as well as private sector, non-government organizations and other stakeholders.
Canada has responded to several chemical events in the past; the Lac Mégantic (2013), the Plastimet fire (1997), the tire fire in Hagersville (1990), the St-Basile-le-Grand polychlorinated biphenyls fire (1988), the Prince Edward Island mussel contamination with domoic acid (1987) and the Mississauga train derailment (1979).
Events where chemicals are mixed together or involved in a fire often represent a public health challenge given the unanticipated by-products that may be generated. This is particularly heightened when the toxicity of the by-products may pose a greater risk the original formulations. Unfortunately, this phenomenon is poorly considered by public health risk assessment or by regulations addressing chemical safety.
The Canadian Disaster Database presents information on other chemical events that occurred in Canada. It contains detailed disaster information on more than 1000 natural, technological and conflict events (excluding war) that have happened since 1900 at home or abroad and that have directly affected Canadians. The Database tracks "significant disaster events" which conform to the Emergency Management Framework for Canada definition of a "disaster" and meet one or more of the following criteria:
- 10 or more people killed
- 100 or more people affected/injured/infected/evacuated or homeless
- An appeal for national and/or international assistance
- Historical significance
- Significant damage/interruption of normal processes such that the community affected cannot recover on its own
The Database describes where and when a disaster occurred, the number of injuries, evacuations, and fatalities, as well as a rough estimate of the costs. As much as possible, the Canadian Disaster Database contains primary data that is valid, current and supported by reliable and traceable sources, including federal institutions, provincial/territorial governments, non-governmental organizations and media sources. Data is updated and reviewed on a semi-annual basis.
Canada has municipal, provincial, territorial and federal capacity to respond to chemical events. These capacities are tested on a regular basis through real and/or simulated events in all provinces and territories and at the national level. Specific components of the response present challenges. Such is the case with roles and responsibilities related to the environmental decontamination of the site in the aftermath of a chemical event. It is unfortunate that this challenge is often only addressed during the later phase of recovery during a chemical event, which leads to confusion and delays. Greater clarity is needed up front to properly define the roles and responsibilities of responding organisations during the recovery phase of a chemical event.
Best practices, challenges, gaps and recommendations
Canada has significant capabilities to respond to chemical events. It is worthwhile to mention best practices and enabling environments that are in place to strengthen capabilities to manage chemical events and areas for improvement.
The Canadian Transport Emergency Centre facilitates communication with appropriate agencies both governmental and private to allow information exchange between appropriate authorities. The Centre's scientific personnel is trained in dangerous goods emergency response and provides technical advice by telephone to responders on site.
The Emergency Response Assistance Plan allows for industry capabilities to be identified in advance of an emergency. These are reviewed by Transport Canada to ensure accuracy and completeness of information based on requirements of the Transportation of Dangerous Goods Regulations.
The Environmental Emergency Regulations favour the detection and response to chemical events by providing an inventory of hazardous sites and facilities. It provides useful information on potential chemical risks and to prepare a proper emergency response.
Challenges, gaps and recommendations
Chemical antidote stockpiles
The creation of a national registry would consolidate a common pan-Canadian toxicological approach for the use of antidotes and stockpiling recommendations while developing a tool for knowledge transfer in the various provinces and territories. The Quebec Poison Control Centre and the National Public Health Institute of Quebec, in partnership with the Health Portfolio and health professionals across Canada, are leading the development of a pilot pan-Canadian antidote registry (similar to the Quebec Antidote Registry) (Available in French only) on the Canadian Network for Public Health Intelligence. The Quebec treatment guide will be adapted to become a national product available in both official languages.
A timely information sharing process about the national availability of chemical antidotes before or during a chemical event is desirable. To date, information about the content of National Emergency Strategic Stockpile is neither routinely shared among federal government departments nor with provincial and territorial jurisdictions.
A more efficient response would result from a better information-sharing process between those jurisdictions that manage a stockpile of chemical antidotes. It would allow for an exchange of best practices between poison centres across Canada and for ready access to critical knowledge and information for an emergency response resulting in better health outcomes.
Standardisation of emergency response training
To ensure that response to chemical events across Canada is uniform, it would be desirable to develop and maintain a standardized training program for first responders and first receivers in regard to hazardous materials and emergency management for chemical events. Currently, it is not mandatory in Canada for first responders and first receivers to follow a specific training program.
Pan-Canadian Surveillance of poison centre data
One other area that is a significant Canadian deficiency is the national aggregation of poison data to facilitate the storage, handling, and rapid analysis of data for surveillance purposes as recommended by the World Health Organization. Canada remains the only G7 country that does not have such a national system.
There are five poison centres across Canada. Most provinces and territories that do not have a poison centre have established agreements for coverage by a neighbouring centre. The main mandate of the poison centre is to provide advice to treat patients exposed to chemicals. Some provinces and territories currently have capabilities for poison centre data surveillance, most do not.
Integration of environmental public health and research components in the response process
It would be beneficial to support the development of a project to integrate the environmental public health and the research components into the response process to a chemical event. Currently, those considerations are not immediately addressed during a chemical event resulting in the loss of useful data to manage the ongoing event and future similar events.
The U.S. Disaster Research Response initiative, led by the National Institute of Environmental Health Sciences and the National Library of Medicine, supports disaster science investigators by offering data collection tools, research protocols, disaster research news and events. Health Canada and the National Collaborating Centre for Environmental Health are co-leading an emerging initiative, supported by the Canadian Network for Public Health Intelligence, to explore the possibility of designing a similar response framework in Canada.
Development of national tools to support the research component
Research activities that support informed decision-making during a chemical event response take time. Delays in getting these activities underway may mean that precious data has disappeared or is no longer available. Therefore, resources and tools to support these activities must be developed, pre-approved and made readily available prior to an event. Canada would benefit from the development of tools similar to the Community Assessment for Public Health Emergency Response (CASPER) toolkit-currently in use in the United States-which provides guidelines on data collection, tool development, methodology, sample selection, training, analysis, and report writing.
Funding and workforce to support the recovery phase of a chemical event
Many of the public health considerations that require significant workforce to support a chemical event occur during the recovery phase. These include environmental sampling and decontamination for re-use or occupancy of the area or property affected. Also, long-term monitoring of the environment and population exposed to monitor health and safety is needed. Greater consideration is needed to establish dedicated contingency funds to address the long term public health needs during the recovery phase. This is a gap which is applicable to all hazards while being of greater contrast for chemical events given the response is often of short duration.
Radiation emergencies
Joint external evaluation target: States parties should have surveillance and response capacity for radio-nuclear hazards/events/emergencies. It requires effective communication and collaboration among the sectors responsible for radio-nuclear management.
Level of capability in Canada
In Canada, ongoing radiation safety is established, maintained and enforced through a mature regulatory and emergency preparedness regime. This regime is fully aligned with international requirements established by the International Atomic Energy Agency and associated international conventions. It is intended to ensure that events of radiological health significance are avoided or minimized both in the accident country as well as in neighbouring states.
While nuclear activities are regulated at the federal level, emergency management in Canada, including for radiation emergencies, is a shared responsibility between the three levels of government (municipal, provincial and territorial, and federal), licensees, and non-governmental organizations in a bottom-up approach.
Most emergencies are local in nature, and managed at the community or provincial and territorial level. The federal government can become involved in support of the provincial/territorial response. It can also step in to address areas of federal jurisdiction, including cross-border impacts and international aspects, particularly under the auspices of two key international conventions: the Convention on Early Notification of a Nuclear Accident and the Convention on Assistance in Case of a Nuclear Accident or Radiological Emergency.
Provincial and territorial governments are responsible for:
- Overseeing public health and safety and protection of property and the environment
- Enacting legislation to fulfill the province's lead responsibility for public safety
- Preparing emergency plans and procedures and providing direction to municipalities that they designate to do the same
- Managing the response to an radiation emergency by supporting and coordinating the efforts of responding organizations
- In the case of nuclear power plants, coordinating support from the licensee and the Government of Canada during preparedness activities and in response to a nuclear emergency
Provinces that operate nuclear power plants or contain ports that berth nuclear power vessels have specific arrangements in place, including nuclear emergency response plans and associated procedures. In jurisdictions where the risk and hazards are lower, preparedness and response to a radiation emergency is based on existing provincial or territorial all hazards plans.
The federal government's involvement in managing potential offsite impacts is required for addressing areas of federal responsibility, including incidents that have an impact beyond provincial or national borders. Likewise, the coordination of federal assistance, when requested by an affected province, is also required.
Some provinces have agreements with the Government of Canada for the provision of specific types of technical support. Federal responsibility also encompasses a wide range of contingency and response measures to prevent, correct or eliminate accidents, spills, abnormal situations and emergencies, and to support provinces and territories in their responses to a nuclear emergency. The Government of Canada is also responsible for:
- Liaison with the international community
- Liaison with diplomatic missions in Canada
- Assisting Canadians abroad
- Coordination of the national response to a nuclear emergency occurring in a foreign country
In accordance with the Emergency Management Act, Public Safety Canada ensures coordination across all federal departments and agencies responsible for national security and the safety of Canadians. It is responsible for coordinating the overall federal government response to emergencies in support of the provinces and territories, including radiological-nuclear emergencies.
Public Safety Canada is the lead authority for the Federal Emergency Response Plan (FERP) which provides an all hazards framework for the response to any emergency requiring a coordinated federal response. Ministers are also responsible for preparing plans to address emergencies that could impact their mandate and responsibilities.
Health Canada is the lead authority for the Federal Nuclear Emergency Plan (FNEP), an event-specific annex to the FERP that provides the supplemental arrangements for managing the radiological health impacts of a nuclear emergency in areas of federal jurisdiction and in support of the provinces and territories. It also has responsibilities related to radiation protection, including cross-Canada monitoring networks, laboratories and decision-support systems.
Health Canada administers a federal interdepartmental and a federal-provincial nuclear emergency management committee, as well as a training and exercise program. Internationally, Health Canada and the Canadian Nuclear Safety Commission serve as national competent authorities to the International Atomic Energy Agency.
The FERP and the related FNEP fully set out the federal framework, roles and responsibilities for management of radio-nuclear events. At the provincial/territorial level, there are similar plans in place to address the varying needs of each jurisdiction. The interface between these two federal plans and provincial and territorial plans provides a consistent and coherent approach for managing radiation emergencies in Canada.
Indicators
RE.1 Mechanisms are established and functioning for detecting and responding to radiological and nuclear emergencies
National policies, strategies or plans for detection, assessment and response to radiation emergencies
Detection, monitoring and notification
In Canada, a public health hazard or emergency posed by a radiation incident, whether originating from natural, technological, accidental or malevolent sources, may be detected by various means through different organizational mandates and capabilities. These means include federal legal/regulatory requirements, routine environmental monitoring, public health surveillance and notification obligations (both nationally and internationally). Environmental radiation monitoring networks and laboratories for monitoring and analysis of radioactivity exist at both the licensee, provincial and federal levels.
There are multi-sectoral stakeholders with roles and responsibilities that are involved in the area of radiation safety in Canada. National policies, strategies and plans for detection, assessment and response are built on the national enabling arrangements as described in the previous section.
At the local level:
-
Licensees are organizations or institutions at the regional or local level who have obtained licenses for radiological or nuclear substances and devices are responsible for on-site surveillance and monitoring of their own activities.
Nuclear facilities, through their licensing conditions, which include site emergency plans, implement arrangements to detect or recognize emergency situations, assess their severity and inform offsite response authorities promptly.
These include routine environmental monitoring programs as well automated real-time station boundary radiation monitoring systems with appropriate backup power and communications systems. Operators are required to provide ongoing information on the progression and severity of the incident to offsite response authorities as well as the regulator.
-
Provinces and territories have primary responsibility for off-site issues, including monitoring and surveillance of radioactivity in the environment. The provincial/territorial governments are responsible for overseeing public health and safety and the protection of property and the environment within their jurisdictions.
Accordingly, they are responsible for the arrangements necessary to detect, assess and respond to the offsite radiological health consequences of a nuclear emergency. They achieve this by enacting legislation and providing direction and guidance to affected communities.
Typically, their administrative structures include an emergency measures organization (or the equivalent) to cope with a wide range of potential or actual emergencies in accordance with the hazard and risk assessment of the jurisdiction and defined plans and procedures for detection and monitoring capabilities.
The provinces maintain emergency operations centres to coordinate the assessment and response actions.
At the federal level:
-
The Canadian Nuclear Safety Commission (CNSC) provides regulatory oversight for on-site activities of licensees, including surveillance/monitoring, detection, assessment and response to a potential radiation emergency.
The CNSC will also receive notification from the licensee of any potential radiation emergency situation according to established triggers. It will undertake an independent assessment of the situation and subsequent response actions as appropriate, including notification to the International Atomic Energy Agency, as set out in the CNSC emergency response plan.
The CNSC supports the FNEP through provision of radiation source term information to inform off-site radiation dose assessment and prognosis. CNSC has also established an Independent Environmental Monitoring Program to confirm that licensees are adhering to regulatory requirements, licence conditions and approved programs throughout the operation of nuclear facilities with respect to releases of radioactive materials to the environment.
The Independent Environmental Monitoring Program consists of sampling environmental media and analyzing radiological and non-radiological substances released from facilities in all areas of the nuclear fuel cycle. These include uranium mines and mills, processing facilities, nuclear power plants, research reactors and waste management facilities.
Samples are analyzed for radiological and non-radiological contaminants at the CNSC's laboratory using industry best practices. The results are compared to appropriate federal and/or provincial guidelines to confirm that the public and the environment around the facility are safe, with no health impacts as a result of facility operations. Conclusions and data are published on the CNSC website and a full technical report is also available upon request.
-
Health Canada maintains a cross-Canada radiation monitoring network and laboratory capability that includes real-time radiation monitoring and reporting and systematic analysis of environmental samples to facilitate rapid detection and assessment of a radiation emergency. This includes a certified laboratory capability at the national level for systematic analysis of environmental samples, as well as monitoring and assessment capabilities as part of the Comprehensive Nuclear Test Ban Treaty International Monitoring System, both of which facilitate detection and assessment of radiation emergencies.
Health Canada's radiation monitoring network laboratories and Comprehensive Nuclear Test Ban Treaty radioisotopes laboratory are both certified (by the International Organization for Standardization and the Comprehensive Nuclear Test Ban Treaty Organization respectively). The laboratories are routinely tested through a constant regimen of official international inter-comparison and validation exercises (Analytical Laboratories for the Measurement of Environmental Radioactivity, the U.S. Environmental Protection Agency, the U.S. Department of Energy, etc.).
Results are published to Government of Canada websites, as well as International Atomic Energy Agency and European Union data platforms. Health Canada also maintains Mobile Nuclear Laboratories that can be deployed to perform radiation surveys, sampling and laboratory analysis in the field. There is also radiobiology laboratory capacity for performing biodosimetry analysis and reference testing.
If Health Canada's capacity is overwhelmed, the Canadian Nuclear Laboratories is available to assist through an informal agreement. Health Canada also operates an occupational radiation dosimetry service as well as the National Dose Registry to monitor the radiation exposure of occupationally-exposed workers.
Health Canada, as lead for the FNEP, will also receive notification of a potential radiological emergency from implicated provinces and territories and the CNSC according to established triggers. Health Canada leads the FNEP Technical Assessment Group to undertake radiation dose assessment and prognosis to support federal situational awareness and provincial or territorial protective action, recommendations, and decisions.
Health Canada is also a designated contact point to receive notification from the International Atomic Energy Agency of an incident outside of Canada that could have an impact on Canada.
- The Department of National Defence/Canadian Armed Forces is responsible for monitoring at ports visited by nuclear powered vessels, and will provide detection and notification of events involving such vessels.
-
The Canadian Food Inspection Agency is responsible for food inspection, with additional analysis by Health Canada where it concerns radiation hazards in foodstuffs. This is conducted through Health Canada's annual total diet study.
Health Canada also provides operational support (analytical and scientific reach back) to the Canadian Food Inspection Agency for radio-analytical capacity according to operational need. Under the FNEP, the Agency has the primary responsibility for assisting in the control of food and goods imported from areas potentially impacted by a radiation emergency.
- Canada Border Services Agency is primarily responsible for Emergency Support Function - Border Services to support International Health Regulations (IHR) requirements in the area of points of entry (Ports, airports, ground crossings).
- Environment and Climate Change Canada provides the national weather prediction service for Canada, including for emergency response purposes. There are formal arrangements between Health Canada and ECCC for the provision of weather data and modeling capabilities and expertise in support of the FNEP.
- Transport Canada notifies the Canadian Nuclear Safety Commission of reportable transport incidents involving radioactive material. A memorandum of understanding between Transport Canada and the Commission outlines the regulatory responsibilities for enforceable actions involving radioactive materials in transport. Transport Canada is the lead for emergency response oversight while the Commission leads on emergencies involving radiological or nuclear materials in transport.
All of the above organizations maintain 24/7 watch officer programs, and there are arrangements between operators, provinces and the federal government for prompt notification in case of a radiation/nuclear emergency. This facilitates timely activation of local, provincial/territorial and federal emergency operations centres, and subsequent response arrangements. However, in a large radiation emergency, the number of implicated response organizations and the rapid timeline of the event may place some challenges on the ability to activate and maintain effective and timely coordination and communication amongst all partners.
The Convention on Early Notification of a Nuclear Accident places specific reporting obligations in relation to a nuclear or radiological emergency of international significance on States parties to the Convention, including Canada, and on the International Atomic Energy Agency. Health Canada and the CNSC are the National Competent Authorities to the Agency for domestic emergencies. CNSC reports on-site conditions and Health Canada reports off-site conditions.
Health Canada is also the focal point for receiving notification of an international radiation emergency of potential radiological significance to Canada. To this end, both organizations and the Government Operations Centre maintain 24/7 duty officer programs for prompt notification.
Radiation emergency response plans
Under the Emergency Management Act, all federal Ministers must plan for emergencies within their areas of responsibility. This includes radiological-nuclear emergencies. In the provinces and territories, there are also Emergency Management Acts, which provide a legal framework that defines responsibility and authorities of each provincial and territorial government when dealing with emergencies within their jurisdiction, such as radiological-nuclear events.
Provincial nuclear plans contain specific details for areas under provincial responsibility, including evacuation. The plans set out in detail the components required to respond to a variety of radiological events. Three Canadian provinces (Ontario, New Brunswick and British Columbia) have specific provincial plans for nuclear emergency response. Provinces and territories with low risk of radiation emergencies either do not possess plans, or revert to an All Hazards plan in conjunction with the federal nuclear response plan.
The Federal Emergency Response Plan is the Government of Canada's all hazards plan, and provides the response framework for any emergency. The Plan is under the responsibility of the Minister of Public Safety Canada.
The Federal Nuclear Emergency Plan integrates with the FERP and describes the Government of Canada's supplemental arrangements for managing a nuclear emergency. It is a multi-departmental, event-specific plan that outlines the roles and responsibilities of federal organizations in preparing for and responding to the radiological health consequences of a nuclear emergency.
The FNEP has provincial annexes for jurisdictions having nuclear power plants or ports visited by nuclear powered vessels. These annexes establish the link between federal and provincial nuclear emergency response organizations and capabilities. The FNEP establishes a Technical Assessment Group whose role is to gather data, conduct assessments and recommend and/or implement appropriate actions for the management of off-site radiological consequences in areas within federal jurisdiction or in support of provinces and territories.
Because of the inherent technical nature and complexity of nuclear emergencies, the FNEP introduces event-specific Nuclear Emergency Functions. These are technical response functions that group actions specifically related to nuclear emergency preparedness and response and that complement the Emergency Support Functions in the Plan.
Responsibilities for each Nuclear Emergency Function are assigned to primary and supporting departments or agencies within the FNEP Assessment Group. Because roles and responsibilities depend upon the specific mandates and capabilities of federal government institutions and the nature of the emergency, functions and assigned departmental responsibilities include, but are not necessarily limited to those identified in the Plan.
All organizations involved in the FNEP are expected to develop their own plans, procedures and capabilities to fulfil their Nuclear Emergency Function responsibilities.
The Health Portfolio Emergency Response Plan is a generic all hazards plan that outlines the operational and planning guidelines required to coordinate the delivery of Health Portfolio support capabilities to provincial, territorial, other federal departments and international partners during an emergency.
The Health Portfolio Emergency Response Plan is augmented by annexes that address specific threats and hazards. One of these is the Nuclear Emergency Response Annex. The Annex is hazard-specific and provides additional details for coordinating in-house radiological/nuclear capabilities. When applicable, the FNEP is triggered in conjunction with the Health Portfolio Emergency Response Plan.
The Nuclear Emergency Response Annex provides linkages between emergency response structures. The Chemical, Biological, Radiological, Nuclear and Explosive (CBRNE) Intentional Events Annex is intended to be used to coordinate a response within the Health Portfolio to an intentional CBRNE event with observed or expected public health impact.
The main role of the federal Health Portfolio during an intentional event emergency is to provide scientific and public health support capabilities to assist response efforts. The Health Portfolio may be required to respond with recommendations for personal decontamination, use of medical countermeasures for treatment and/or prophylaxis, provision of mass prophylaxis, isolation and quarantine, and sheltering and evacuation.
At the provincial/territorial level, provinces that operate nuclear power plants or contain ports that berth nuclear power vessels have specific arrangements in place, including specific nuclear emergency response plans and associated procedures. For example, Ontario maintains a Provincial Nuclear Emergency Response Plan and implementation plans for each nuclear power plant, as well as a plan for other radiological emergencies. Ontario has also developed a Radiation Health Emergency Plan. New Brunswick has a Provincial Health Nuclear Emergency Plan for the Point Lepreau Generating Station. In jurisdictions where the risk and hazards are lower, preparedness and response to a radiation emergency is based on existing provincial or territorial all hazards plans.
In the provinces and territories, some jurisdictions have established coordination between the health system, emergency medical services and other key stakeholders. For instance, the Manitoba health system has some linkages with police and fire/EMS programs, as well as municipal emergency programs, CancerCare Manitoba Radiation Protection, and the University of Manitoba.
In provinces that have reactor facilities, the provincial plans, their associated hazard assessments and their response capabilities link directly to the FNEP and that of the facility operators. The operators' onsite emergency plans and associated provisions for a response provide the framework for interaction with external authorities and serve as the basis for event preparedness and response. The hazard addressed in the planning may be caused by an accident, malfunction or loss of control involving radioactive material from a major nuclear facility or it may result from other sources.
Dispersal of radioactive contaminants as a result of a malicious act, such as a dirty bomb, would invoke existing consequence management plans and resources for radiation protection, as would other radio-nuclear emergencies.
Response plans and procedures are periodically reviewed to incorporate lessons learned from exercises and real events to ensure that they remain fit for purpose. For example, following the Fukushima Daiichi nuclear accident in Japan in 2011, the FNEP was updated and provincial plans have either been updated or are in the process of being updated.
Technical guidelines for managing radiation emergencies
There are operating procedures and technical guidelines in place for the management of radiation emergencies, including arrangements and procedures for surveillance/monitoring, risk assessment, reporting, event confirmation, and notification to trigger an appropriate response when required.
Escalation procedures at all levels are described in the emergency plans of facilities, provinces and territories, and federal organizations, including the provincial nuclear emergency plans and the FNEP and its supporting documents. The FNEP is triggered when there is potential for a release of radioactivity from a source, or based on data from real-time radiation monitoring networks.
The triggers for the Health Portfolio Emergency Response Plan Nuclear Emergency Response Annex include the emergence of significant public exposures to radiation (possibly of unknown source) requiring immediate coordinated response within the Health Portfolio. The Annex may be triggered without an FNEP escalation.
In event of a nuclear power plant accident, provincial and federal Technical Assessment Groups and associated operating procedures bring together the scientific capabilities that reside across the various government organizations to assess potential radiological consequences and propose recommendations on urgent protective actions to decision makers. These activities are supported by concepts of operation, technical manuals and standard operating procedures. Specific functions include radiation source term assessment, exposure pathways modeling, environmental monitoring and assessment, human monitoring, medical response and public communications.
The FNEP Technical Assessment Group Manual defines the roles and responsibilities of the individuals responding to a radiation emergency under the FNEP (note these individuals may be from 18 departments/agencies identified in the FNEP and work closely with provincial counterparts). The manual is supported by standard operating procedures for specific tasks.
The FNEP Technical Assessment Group Manual, supported by the FNEP Technical Assessment Group's Risk Assessment Group's concept of operations and standard operating procedures, provides the guidance and tools to support the development of advice and recommendations for jurisdictionally-based (federal, provincial) decision makers. For non-FNEP radiation emergencies, Health Canada would use best practices to support the development of advice and recommendations for jurisdictionally-based (federal, provincial) decision makers. Primary responsibility for restrictions/evacuation resides with the provinces and territories.
Canada has developed intervention levels for various protective actions. Criteria for protective actions, including evacuation, sheltering, iodine thyroid blocking, and relocation, are written into provincial nuclear plans. There is also specific federal guidance. Federal guidance specific to radiological emergency response includes the Generic Criteria and Operational Intervention Levels for Nuclear Emergency Planning and Response (2017), which provides recommendations on radiation quantities to trigger implementation of protective action strategies during an emergency, for the public and for off-site workers, as well as guidance for their use in planning. This guidance was developed in close consultation with provincial stakeholders and is incorporated in the latest provincial nuclear plans to facilitate harmonization across jurisdictions.
The Canadian Guide on Medical Management of Radiation Emergencies (2015) provides information to medical responders and hospital personnel on screening, assessment and emergency room treatment of populations exposed to radiation or contaminated by radioactive materials.
Canada has developed emergency bioassay methods for rapidly measuring strontium-90, radium-226, polonium-210 (in development), plutonium-239/240, and americium-241. There are protocols in place for coding and managing cases and standard operating procedures for biodosimetry methodology for emergency response. The Medical Emergency Treatment for Exposure to Radiation training course is also available to first receivers.
International treaties, protocols and standards
Canada is signatory to the following treaties:
- Convention on Early Notification of a Nuclear Accident
- Convention on Assistance in the Case of a Nuclear Accident or Radiological Emergency
- Convention on Nuclear Safety
- Convention on the Physical Protection of Nuclear Material
- Amendment to the Convention on the Physical Protection of Nuclear Material
- Joint Convention on the Safety of Spent Fuel Management and on the Safety of Radioactive Waste Management
- Convention on Supplementary Compensation for Nuclear Damage
- Comprehensive Nuclear Test Ban Treaty (CTBT)
The Convention on Early Notification of a Nuclear Accident places specific reporting obligations in relation to a nuclear or radiological emergency of international significance on States parties to the Convention, including Canada, and on the International Atomic Energy Agency. Health Canada and the CNSC are the National Competent Authorities to the Agency for domestic emergencies. CNSC reports on-site conditions and Health Canada reports off-site conditions.
Health Canada is also the focal point for receiving notification of an international radiation emergency of potential radiological significance to Canada. To this end, both organizations and the Government Operations Centre maintain 24/7 duty officer programs for prompt notification.
Decontamination of people, premises and environment
Provincial nuclear plans contain arrangements for monitoring and decontamination centres. These arrangements include procedures for radiation screening, decontamination, and medical follow-up, if necessary. Protocols exist for deploying federal assets, including equipment and expert personnel, to manage decontamination following a radiation emergency. Recommendations for decontamination and follow-up are identified in the Generic Criteria and Operational Intervention Levels for Nuclear Emergency Planning and Response (2017). The number of available, expert staff is small, and therefore the capability is designed to integrate into monitoring and decontamination units run by local authorities.
Ontario's Radiation Health Response Plan (2014) describes the locations where people can be decontaminated (site of incident, hospital, home, monitoring and decontamination unit). As another example, the New Brunswick Power Nuclear Emergency Response Plan, an all hazards, onsite emergency plan for Point Lepreau, supports the offsite provincial plan in all aspects, including a mass decontamination plan, which details requisite monitoring and decontamination in the event that a nuclear emergency requires evacuation of local area residents. The emergency organization and tools outlined in the plan are designed around the requirement for interoperability with provincial and local emergency management partners, as well as with federal agencies. Outside of areas with nuclear facilities, the local arrangements are of a much lower capacity.
The FNEP contains a list of Responsibilities of Designated Federal Government Institutions for Nuclear Emergency Functions. It should be noted however that the FNEP covers only the consequence management phase and not the recovery phase. Currently there is limited Canadian guidance regarding the decontamination of premises and environment. Canada has been working on a framework to address the issue but there is more work to do in this respect.
Arrangements for decontamination of the environment as part of longer term recovery are less well developed, with known gaps. Work is in progress to develop a framework for recovery, including clarifying functional needs, roles, responsibilities and capabilities.
Systematic information exchange between radiation safety and health authorities
The national emergency preparedness and response framework establishes an integrated response structure across all jurisdictions and organizations, including radiation competent authorities (CNSC and Health Canada) and health authorities (provincial health authorities, PHAC, Health Canada). This enables the systematic and timely exchange of information between these authorities about detection, notification and response for radiological emergencies of potentially international concern.
Additionally, Health Canada, as lead for the FNEP and National Competent Authority to the International Atomic Energy Agency for the two international nuclear emergency conventions on notification and assistance, has established a protocol with Canada's IHR National Focal Point within the Public Health Agency of Canada to ensure exchange of information, and coordination of reporting for events of international significance to International Atomic Energy Agency and the World Health Organization.
Mechanisms to access health facilities
A number of reference health care facilities across the country are equipped to deal with radiation emergencies. However, since this type of preparedness is generally based on hazard and risk assessment, jurisdictions where there are no nuclear facilities typically will have lower capacity to respond to radiation emergencies.
Hospitals
Hospitals play a role according to established nuclear emergency plans. Provincial nuclear emergency plans designate certain hospitals for the response to a nuclear emergency resulting from an incident at a nuclear facility. For example, in New Brunswick, according to the Provincial Health Nuclear Emergency Plan for the Point Lepreau Generating Station, Saint John Regional Hospital is the designated health care facility for the Point Lepreau Generating Station during an on-site or off-site event.
Ontario's Radiation Health Response Plan (2014) describes the role of hospitals, identifies four designated hospitals in Ontario that are close to nuclear installations and describes baseline expectations. Ontario hospitals that provide emergency care are equipped with a standardized package of supplies and equipment as part of the Provincial CBRN Emergency Preparedness Program. There are also a number of hospitals in major population centres that would be capable of receiving an exposed and/or contaminated patient.
In another example, five of six hospitals in Winnipeg (Manitoba) have radioactive activities, are licensed by CNSC and are part of the regional radionuclide safety program. All emergency rooms in Winnipeg have been provided with a standard operating procedure on radionuclide therapy for patients seeking emergency care.
A training course on Medical Emergency Treatment for Exposure to Radiation, delivered by Health Canada and partners, is also available to first receivers, and has been delivered at locations across the country. An on-line version is also available. Finally, additional support may be requested under the Convention on Assistance in Case of a Nuclear Accident or Radiological Emergency.
Referral, transport and treatment of large numbers of affected individuals
Plans for the transport and treatment of affected individuals do not exist in all jurisdictions where there is low risk of radio-nuclear accidents. Some jurisdictions have incorporated this to a certain extent in emergency management services planning. For instance, Alberta has plans for mass casualty incidents. All emergency departments and urgent care centers in Alberta are familiar with it and staff are trained to respond to a Code Orange (mass casualty) or Code Brown (hazardous material). Alberta Health Services has a Referral, Access, Advice, Placement, Information and Destination call centre.
The FNEP and supporting documents do not include special plans for referring, transporting, or treating large numbers of individuals because it is considered extremely unlikely that many, if any, would be exposed to levels of radioactivity that would pose a non-stochastic health risk. It is much more likely that large numbers of individuals could require referral and treatment for psychosocial effects as a result of fear during the emergency and disruptions to their lives as a result of the response. There are no protocols specific to radiation emergencies for this at this time.
Under Nuclear Emergency Function 7 "Medical Response," the FNEP includes two activities:
- Provide training for the treatment of contaminated and/or overexposed casualties
- Provide or arrange for the provision of medical radiation expertise and for the treatment of contaminated and/or overexposed casualties.
Follow-up of patients would generally be a provincial or territorial responsibility.
Health Canada manages the provision of biological dosimetry for affected individuals as part of the FNEP Human Monitoring Technical Assessment Group team. Procedures and standard operating procedures are in place for performing this response. The FNEP Technical Assessment Group Biodosimetry Task Team is ready to receive 100 blood samples at any time. Preliminary reports should start to be available about 60 hours after receipt of blood samples at the laboratory, with all samples being analysed within 96 hours.
Equipment and medication stockpiling
The provision of medical care, including the medical response to a nuclear emergency, primarily falls under provincial and territorial jurisdiction. Potassium iodide (KI), to prevent radiation-induced thyroid cancer, is pre-distributed to all households, business, and public facilities in the emergency planning zones around all nuclear power plants.
The Health Portfolio maintains limited supplies of medical countermeasures for internal radiological contamination in the National Emergency Strategic Stockpile. Upon request by appropriate medical or public health authorities, these supplies can be made available to provincial/territorial authorities for use in their response to a nuclear emergency. Examples of medical countermeasures in the National Emergency Strategic Stockpile include, Prussian Blue, Calcium- and Zinc-DTPA, and potassium iodide (KI).
Public communication
Informing the public is one of the required activities under the FNEP. As per the FERP, federal communications are managed by Public Safety Canada. The plan also states that in accordance with existing provincial nuclear emergency plans, provincial/territorial information centres will be the main source of public and media information on aspects of emergency operations and protective actions.
The FNEP Federal Spokesperson(s) will present the federal position concerning the nuclear emergency in coordination with the provincial information centres. The federal Chief Public Health Officer has a specific role to communicate information to the public in the event of a radiation emergency with potential radiological health impacts.
At the provincial/territorial level, New Brunswick and Ontario provide information to the public as outlined in their provincial plans. In New Brunswick there are plans and procedures in place that support government communications and the provision of public information during emergencies.
The New Brunswick Emergency Measures Organization has the capability to notify residents in the Emergency Planning Zone by means of a mass notification system. In Ontario, the Provincial Nuclear Emergency Response Plan outlines the requirements for a program of Public Education for Nuclear Emergencies. The program aims to educate people living or working in the Primary Zones of nuclear installations about the actions they should take to protect themselves before or during a nuclear emergency (see section R5: Risk communication).
RE.2 Enabling environment is in place for management of radiation emergencies
Coordination and communication between national authorities for radiological and nuclear events and health authorities
Nationally, planning for radiation emergencies is aligned and integrated with all hazards arrangements. Plans at the federal and provincial levels describe the inter-jurisdictional coordination and communication mechanisms. These include the FERP, the FNEP, and provincial plans. The roles of all relevant organizations, including links between licensee, regulatory, response and health authorities are clearly elaborated, including designated focal points.
Health Canada, as lead for FNEP and National Competent Authority to the International Atomic Energy Agency for the two international nuclear emergency conventions on notification and assistance, has established a protocol with Canada's IHR National Focal Point to ensure exchange of information, and coordination of reporting for events of international significance to the International Atomic Energy Agency and World Health Organization. Health Canada and the Public Health Agency of Canada are both within the federal Health Portfolio, which facilitates coordination, communication and preparedness activities for radiation emergencies.
As described under indicator RE.1, the FNEP is a multi-departmental plan that outlines the roles and responsibilities of federal organizations in preparing for and responding to the radiological health consequences of a nuclear emergency. The FNEP integrates with the FERP and the National Emergency Response System to provide a coherent federal-provincial-territorial coordination and communication structure for response to a radiation emergency.
The provinces and territories, which maintain their own nuclear emergency or all hazards plans according to risk, have their own emergency management structures and requirements for federal support in the event of a radiation emergency. Some jurisdictions have also incorporated the key federal organizations directly in their nuclear or all hazard response plans. These might include:
- Public Safety Canada
- Health Canada
- Canadian Nuclear Safety Commission
- Public Health Agency of Canada
- Canadian Food Inspection Agency
In the provinces and territories, there are a variety of coordination mechanisms in place. Some jurisdictions have established plans, capacity and processes to interface across sectors in a radiation emergency while other have limited capacity, however, this generally reflects the relevant hazard assessment.
Coordination between jurisdictions and organizations is built on existing all hazards arrangements, supplemented with event-specific arrangements as required. One example of this type of coordination is the links between CNSC, Health Canada, the Public Health Agency of Canada and provincial authorities.
To facilitate inter-jurisdictional coordination and communication, FNEP provincial annexes have been developed for jurisdictions having nuclear power plants or ports visited by nuclear powered vessels. These annexes describe the coordination between the FNEP and provincial plans, including linkages between the federal and provincial technical assessment groups. Arrangements are in place to mobilize federal resources to meet additional demands. These resources include liaison officers, technical experts, and radiation monitoring. Support to provinces and territories without annexes follow the established arrangements of the FERP.
There are federal, federal/provincial/territorial, and provincial/territorial committees to facilitate and strengthen coordination and communication between authorities. For example, the FNEP is supported by two standing nuclear emergency preparedness advisory committees:
- The Interdepartmental Radiological-Nuclear Emergency Management Coordinating Committee
- The Federal/Provincial/Territorial Radiological-Nuclear Emergency Management Coordinating Committee
Additionally, Health Canada, as lead for FNEP and National Competent Authority to the International Atomic Energy Agency for the two international nuclear emergency conventions on notification and assistance, has established a protocol with Canada's IHR National Focal Point within the Public Health Agency of Canada to ensure exchange of information, and coordination of reporting for events of international significance to the International Atomic Energy Agency and the World Health Organization. The fact that Health Canada and the Public Health Agency of Canada are both within the federal Health Portfolio facilitates coordination and preparedness activities for radiation emergencies.
Radiation emergency plan and transportation of radioactive materials
While there is no single overarching national radiation plan, there is a national framework. As such, all relevant plans at the federal, provincial and local levels follow a common approach, are inter-operable. The various plans for radiation emergencies are aligned with the International Atomic Energy Agency safety standard for Preparedness and Response for a Nuclear or Radiological Emergency. This standard, which has also been co-sponsored by the World Health Organization, covers general requirements, functional requirements and infrastructure requirements for all bodies involved in the national radiation emergency response systems.
In Canada, the packaging and transport of radioactive materials is part of the regulatory framework established by the CNSC. All nuclear substances are transported in packages that are selected based on the nature, form, and quantity or activity of the substance. There are general design requirements that apply to all package types to ensure that they can be handled safely and easily, secured properly, and are able to withstand routine transport conditions.
The CNSC issues licences and certificates for certain kinds of packaging and transport of nuclear substances as stipulated in the Packaging and Transport of Nuclear Substances Regulations (2015). These regulations are based on the International Atomic Energy Agency Regulations for the Safe Transport of Radioactive Material.
The Canadian Transport Emergency Centre is a national advisory service available to assist emergency response personnel in handling dangerous goods emergencies on a 24/7 basis. Some radioactive materials require an emergency response assistance plan during transport, which is required by the Transportation of Dangerous Goods Regulations for dangerous goods for which special expertise and response equipment is required in order to respond to an incident. The plan is intended to assist local emergency responders by providing them with technical experts and specially trained and equipped emergency response personnel at the scene of an incident.
Remedial measures specialists are also available during emergencies to provide advice and on-site attendance on transportation of dangerous goods emergency response procedures.
As part of the CBRNE Resilience Strategy for Canada, Transport Canada may, under certain circumstances, engage emergency responders from the private sector to assist and deal with radioactive materials involved in a terrorism event. Refer to section R3: Linking public health and security authorities for more information on the CBRNE Resilience Strategy. Paragraph 7.1(b) of the Transportation of Dangerous Goods Act allows Transport Canada to authorize the implementation of an ERAP for dangerous goods events in which the provenance of the goods is unknown. In accordance with the FERP during an emergency, Transport Canada provides technical, emergency response, and regulatory advice related to the transportation of dangerous goods.
Canada is bound by the following international treaties for marine and aviation transport:
- International Convention for the Prevention of Pollution from Ships
- International Convention for the Safety of Life at Sea
- Convention on International Civil Aviation (also known as Chicago Convention)
International transport of dangerous goods by marine and air modes must be in accordance with the International Maritime Dangerous Goods Code and the Technical Instructions for the Safe Transport of Dangerous Goods by Air, respectively.
At the provincial/territorial level, there are also plans for transport of radioactive material, samples and waste management including those from hospitals, according to legislated requirements. For example, in Ontario the Provincial Nuclear Emergency Response Plan - Other Radiological Emergencies Plan covers incidents related to transport of radioactive material. The Radiation Health Response Plan covers all radiological and nuclear hazards including the disposal of contaminated items.
Multi-sectoral coordination and communication for radiation safety
In Canada, ongoing radiation safety and emergency preparedness is established, maintained and enforced through a mature regulatory and emergency preparedness regime, which is fully aligned with international requirements established by the International Atomic Energy Agency and associated international conventions. This includes mechanisms for effective coordination and communication between national competent authorities responsible for nuclear regulation, health, and other sectors, as described above. The regime is intended to ensure that events of radiological health significance are avoided or minimized both in the accident country as well as in neighbouring states.
In the provinces and territories, there are a variety of coordination mechanisms in place. Some jurisdictions have limited capacity (training, equipment, etc.) to respond to radio-nuclear events. Therefore, coordination with the federal level would be required. Other jurisdictions have established plans, capacity and processes to interface across sectors. For instance, Alberta takes an all hazards approach to emergency management events. The provincial Emergency Management Agency is the single point of contact to coordinate requests throughout the province. The Radiation Protection Act outlines the process for notifying the appropriate individuals in case of an incident and or overexposure occurring. In the event of a radiation emergency, necessary resources at the federal level can be mobilized to meet additional demands.
Below is a more detailed list of multi-sectoral coordination mechanisms, based on key functional areas.
Public Health
At the federal level:
- The Federal/Provincial/Territorial Radiation Protection Committee supports federal, provincial and territorial radiation protection agencies in their respective mandates. The Committee's mandate is heavily focused on promoting and improving radiation protection standards and programs from a public health standpoint.
- A memorandum of understanding between Health Canada and the CNSC (renewed 2017) outlines agreed-upon activities related to the health aspects of radiation safety, including but not limited to:
- Cooperation and exchange of information with CNSC on occupational and public health matters and health risks associated with the development, production and use of nuclear energy and nuclear substances, and radiation emitting medical devices
- Development of standards for internal dosimetry services to assess internal radiation doses of workers of the CNSC's licensees
- Assistance in reviewing and providing input to epidemiological studies assessing the relationship between ionizing radiation and health effects
- Advancing the development and harmonization of practices and standards for radiation protection within Canada, in accordance with the Terms of Reference of the Federal/Provincial/Territorial Radiation Protection Committee
- Coordinating on-site and off-site activities during an emergency at a Canadian nuclear power plant
At the provincial and territorial level, coordinating bodies also exist, particularly in jurisdictions where there is a higher risk of radio-nuclear events. For instance, in New Brunswick there is a Nuclear Control Group composed of members from organizations that have a role and/or expertise in a nuclear emergency response. The Nuclear Control Group assumes control, direction and coordination of emergency off-site activities.
Ontario has a Nuclear Emergency Management Coordinating Committee, comprised of federal, provincial, regional partners and nuclear power plant. The objective of the committee is to achieve, maintain and enhance an adequate level of preparedness and coordination between provincial ministries, federal departments, nuclear facilities, designated municipalities and other agencies to effectively respond to nuclear and radiological emergencies.
At the international level:
- International Atomic Energy Agency Emergency Conventions: Health Canada is the National Competent Authority to the Agency for the Convention on Early Notification of a Nuclear Accident, and the Convention on Assistance in Case of a Nuclear Accident or Radiological Emergency. Heath Canada contributes to the Agency's Response and Assistance Network.
- Health Canada and the U.S. Department of Energy developed a statement of intent supporting joint Canada-U.S. nuclear emergency preparedness and response capabilities. The statement aims to identify areas where coordination and cooperation between the two countries is needed for nuclear emergency management programs and capabilities. This includes information sharing and mutual assistance.
- Global Health Security Initiative Radiological-Nuclear Working Group: Health Canada/Radiation Protection Bureau participates on this working group of radiological health authorities from the G7 + Mexico.
Environment
The provincial/territorial governments are responsible for overseeing public health and safety and the protection of property and the environment within their jurisdictions. Accordingly, they assume lead responsibility for the arrangements necessary to respond to the offsite effects of a nuclear emergency and outline early protective actions within their provincial plans. Supporting this at a federal level is a memorandum of understanding between Health Canada and Environment and Climate Change Canada for the provision of weather data and modeling capabilities and expertise in support of the FNEP.
Nuclear power plants
Nuclear power plant operators are members of provincial emergency management organizations in Ontario and New Brunswick. They also report to the CNSC with regard to their activities in responding to a nuclear emergency on-site.
Emergency preparedness training and exercises
Canada maintains a nuclear emergency training, drill and exercise programme that includes development and implementation of a long-term program for training, drills and exercises including participation in federal, provincial/territorial, and international drills and exercises. This includes those organized under the International Atomic Energy Agency (ConvEx exercises) and the Organization for Economic Co-operation and Development Nuclear Energy Agency (INEX exercises).
The CNSC requires its licensees to routinely exercise their on-site plans, including the interface with off-site authorities. Targeted drills and exercises are conducted on a routine basis, and large-scale multi-jurisdictional exercises occur in general every two to three years. Each exercise and drill is followed by an after-action report and a management action plan. This was also completed after the Fukushima Daiichi nuclear accident in 2011. These reports and plans integrate relevant lessons learned into the plans and procedures and are re-exercised to increase the level of preparedness.
Examples of recent exercises include:
- 2011 INEX 4 with the Organization for Economic Co-operation and Development Nuclear Energy Agency
- 2012 RadEx for Global Initiative to Counter Nuclear Terrorism
- 2014 Unified Response in Ontario (national full-scale exercise involving licensee local/provincial/federal levels and the International Atomic Energy Agency)
- 2015 Exercise Intrepid in New Brunswick (national exercise involving licensee local/provincial/federal levels and the International Atomic Energy Agency)
- 2016 Exercise Huron Resolve in Ontario
- 2017 Staunch Maple exercise with DND in Nova Scotia
Finally, nuclear safety and security falls under a well-established international framework, of which the International Atomic Energy Agency is a key stakeholder. The Agency offers a peer review service that includes nuclear preparedness arrangements (Emergency Preparedness Review) against relevant international standards. As part of continuous improvement, Canada has invited the International Atomic Energy Agency to organize an international peer review of Canada's national nuclear emergency preparedness arrangements with a target date of June 2019, focusing on events at nuclear power plants.
Best practices, challenges, gaps and recommendations
Canada's emergency response system for radiation emergencies is well established and tested, with clear roles, responsibilities and authorities identified for key stakeholders. Exercises of events at nuclear power plants have demonstrated that plans are fit for purpose and inter-operable. However, it is recognized that in a large radiation emergency, the number of implicated response organizations and the rapid timeline of the event may place some challenges on the ability to activate and maintain effective and timely coordination and communication amongst all partners.
While roles and responsibilities are clear, the level of capability and preparedness in organizations and jurisdictions across the country are not fully evident and varies greatly. As expected, those jurisdictions with nuclear facilities possess greater capacity for response to radiation emergencies. Note that these types of emergencies have the greatest potential to lead to an event of international concern. Outside of these areas, the capacity to respond at the local level is limited, particularly for threats involving the deliberate use of radiation dispersal devices such as dirty bombs.
Though the FNEP has been endorsed at the Deputy Head level by all participating federal organizations, it is not legislated. As a result, the extent to which federal departments are prepared to fulfil their assigned FNEP Nuclear Emergency Functions in terms of resources, responsibilities and tasks is not explicitly mandated. This leaves open to interpretation the extent of delivering on the Nuclear Emergency Functions.
The National Emergency Strategic Stockpile maintains limited supplies of medical countermeasures for internal radiological contamination. There is not a clearly defined prioritization for the distribution of these counter measures (for first responders, federal workers or general public). It is recommended that a study be carried out to determine the potential number of internally contaminated persons based on the hazard and risk assessment, and procure the counter measures accordingly.
Canada has the capacity to respond to radiation events at the outset. However, the availability of resources during an extended emergency would become an issue and there may be a need to approach international partners for support.
The recovery period, though not explicitly within the scope of the IHR, is an area where additional focus is needed to further clarify roles and responsibilities and ensure adequate resources. Training to increase awareness of staff involved in recovery efforts is also essential. At the national level there is no department identified for leading the planning activities about decontamination and radioactive waste management. However, the CNSC recently published a discussion paper aiming at establishing a framework for recovery following a nuclear or radiological emergency, including principles for the management of contaminated waste resulting from an emergency. There is still more work to be done in this area.
It is recommended that continued collaboration between all levels of government be undertaken to further strengthen Canada's capacity to respond to radiation emergencies. A key step would include a thorough analysis of available capabilities and levels of preparedness across jurisdictions to identify areas for improvement and opportunities to provide support.
It should also be noted that while arrangements exist for managing the radiological health aspects of radiation emergencies, a major component to consider is the psycho-social impact, for which there are recognized gaps and challenges.
Appendix 1: Abbreviations and acronyms
- AMR
- antimicrobial resistance
- AMU
- antimicrobial use
- BSI
- blood stream infection
- CAF
- Canadian Armed Forces
- CANUS
- Canada - United States
- CANUTEC
- Canadian Transport Emergency Centre
- CBRNE
- chemical, biological, radiological, nuclear and explosives
- CBS
- Canadian Biosafety Standard
- CBSA
- Canada Border Services Agency
- CDC
- Centers for Disease Control and Protection
- CFIA
- Canadian Food Inspection Agency
- CIHR
- Canadian Institutes of Health Research
- CIPHI
- Canadian Network for Public Health Intelligence
- CNDSS
- Canadian Notifiable Disease Surveillance System
- CNPHI
- Canadian Network for Public Health Intelligence
- CNSC
- Canadian Nuclear Safety Commission
- CPIP
- Canadian Pandemic Influenza Plan
- DND
- Department of National Defence (Canada)
- DTPA
- diethylentriamene pentaacetate
- ECCC
- Environment and Climate Change Canada
- EIOS
- Epidemic Intelligence from Open Source
- ERAP
- emergency response assistance plan
- EURDEP
- European Radiological Data Exchange Platform
- FERP
- Federal Emergency Response Plan
- FNEP
- Federal Nuclear Emergency Plan
- GMP
- good manufacturing practices
- GPHIN
- Global Public Health Information Network
- HPOC
- Health Portfolio Operations Centre
- HPTA
- Human Pathogens and Toxins Act
- IHR
- International Health Regulations (2005)
- IMDG
- International Maritime Dangerous Goods Code
- IMPACT
- Immunization Monitoring Program ACTive
- INFOSAN
- International Food Safety Authorities Network
- IRMIS
- International Radiation Monitoring Information System
- ISO
- International Organization for Standardization
- JEE
- Joint External Evaluation
- MCM
- medical countermeasures
- MRSA
- methicillin-resistant Staphylococcus aureus
- MSSS
- Ministère de la Santé et des services Sociaux de Québec
- NAPAPI
- North American Plan for Animal and Pandemic Influenza
- NESS
- National Emergency Strategic Stockpile
- NFP
- National Focal Point
- NML
- National Microbiology Laboratory
- PHAC
- Public Health Agency of Canada
- PHN
- Public Health Network
- PPE
- personal protective equipment
- RCMP
- Royal Canadian Mounted Police
- SARS
- severe acute respiratory syndrome
- TEPHINET
- Training Programs in Epidemiology and Public Health Interventions Network
- U.K.
- United Kingdom
- U.S.
- United States
- VRE
- vancomycin-resistant Enterococcus
- WHIMIS
- Workplace Hazardous Materials Information System
- WHO
- World Health Organization
Appendix 2: Acknowledgements
The Canadian Government's Joint External Evaluation Project Team gratefully acknowledges the enormous contribution to Canada's self-assessment from the individuals and organizations listed here:
Provincial and territorial government organizations
- British Columbia Provincial Health Services Authority, Health Emergency Management BC
- Alberta Health, Health Protection Branch
- Manitoba Health, Public Health and Primary Care
- Saskatchewan Ministry of Health, Health Emergency Management Unit
- Ontario Ministry of Health and Long-Term Care, Emergency Management Branch
- Quebec Ministry of Health and Social Services
- New Brunswick Department of Health, Emergency Preparedness and Response Branch and the Office of the Chief Medical Officer of Health
- Prince Edward Island Department of Health and Wellness
- Nova Scotia Department of Health and Wellness, Health Services Emergency Management
- Newfoundland and Labrador Department of Health and Community Services
- Northwest Territories Department of Health
- Government of Nunavut
- Yukon Emergency Services
Government of Canada contributing departments
- Public Health Agency of Canada
- Health Security Infrastructure Branch
- Infectious Diseases and Prevention Control Branch
- Office of International Affairs for the Health Portfolio
- Health Canada
- First Nations and Inuit Health Branch
- Communications and Public Affairs Branch
- Healthy Environments and Consumer Safety Branch
- Canadian Food Inspection Agency
- Office of Emergency Management, Policy and Programs, Vice-President Operations
- Canadian Wildlife Service
- Environmental Protection Branch, Environment and Climate Change Canada
- Transport Canada
- Civil Aviation
- Transport of Dangerous Goods
- Canada Border Services Agency
- Canadian Armed Forces
- Royal Canadian Mounted Police
- Migration Health, Immigration, Refugees and Citizenship Canada
- Public Safety Canada
Self-assessment report technical leads
- Katharine Acs-Charter
- Brian Ahier
- Peter Buck
- Tammy Delaney-Plugowski
- Lisa Fillips
- Christine Gagnon
- Theodore Kuschak
- Melanie Kirkey
- Melissa Lee
- Stacey Mantha
- Russell Mawby
- Scott McGinnis
- Lynn Menard
- Sean Monaghan
- Dominique Nsengiyumva
- Stephen Parker
- Jennifer Raven
- Erin Schock
Committees and groups
- International Health Regulations Health Portfolio Working Group
- Public Health Emergency Managers Task Group
Appendix 3: Other relevant documents
P1: National legislation, policy and financing
- Health Portfolio Emergency Response Plan
- Memorandum of understanding between the Public Health Agency of Canada and the Department of National Defence related to inspection and issuing of ship sanitation control certificates and ship sanitation control exemption certificates for Canadian Armed Forces vessels
- PHAC, HC, CFIA joint protocol for IHR communications related to food safety issues
- Strategic Emergency Management Plan
P2: International Health Regulations coordination, communication and advocacy
- Guidebook for IHR Assessment and Reporting at the Federal Level
- Guideline for sharing International Health Regulations notifications of events in Canada with the Council of Chief Medical Officers of Health and IHR Champions
- Strategic overview of Canada's IHR National Focal Point
P3: Antimicrobial Resistance
- Canadian Antimicrobial Resistance Surveillance System 2017 Report (PHAC, 2018)
P4: Zoonotic disease
- Assessment of non-enteric zoonotic diseases of public health significance with the highest risk of emergence or re-emergence in Canada , 2012 (PHAC)
- Letter of Agreement Regarding Zoonotic Surveillance and Risk Assessment Process (2008)
- Memorandum of Understanding for Common Issues Related to Human Health (2008)
- Situational awareness report on the first recorded human case of locally-acquired eastern equine encephalitis in Ontario in late 2016
- Performance of Veterinary Services Evaluation for Canada
P5: Food safety
- Federal Food Safety Communications Protocol for National Food-Borne Illness Outbreaks (2005)
- Guidance for the use of whole genome sequencing in developing countries (PHAC)
- Joint protocol for sharing information between the Health Portfolio and international partners
- Memorandum of Understanding between Health Canada and the Canadian Food Inspection Agency regarding roles during an event or emergency (1999)
- Memorandum of understanding between Health Canada, PHAC and the Canadian Food Inspection Agency describing roles and responsibilities for common issues that impact human health (2008)
- Program Management Framework (CFIA)
- Roles and Responsibilities Framework for Federal Food Safety and Inspection Activities (June 1999)
P6: Biosafety and biosecurity
- Safety and Risk Management Policy (Manitoba)
- National Microbiology Laboratory Incident Response Manual
- National Microbiology Laboratory Biosafety Manual
- Vaccination policy for laboratory personnel (New Brunswick)
- Stanton Territorial Health Authority Biosafety Program Manual (Northwest Territories)
- Recommended Immunization Schedule for Health Care Workers (Prince Edward Island)
D1: National laboratory system
- Biosecurity Plan
- Canadian Public Health Laboratory Network Strategic Plan 2016-2020
- Canadian Public Health Laboratory Network Core Functions of Health XWalk 2011 V2.2
- Plan for Administrative Oversight
- Standard operating procedures
D2: Real-time surveillance
- Directive for the Collection, Use and Disclosure of Information Relating to Public Health (PHAC, 2013)
D3: Reporting
- 2016 Privacy Impact Assessment: IHR National Focal Point
- Flowchart for IHR Assessment and Reporting (approvals) - Article 6
- Guidebook for IHR Assessment and Reporting at the Federal (national) Level
- Guideline for Internal/External Communication of an International Health Regulations (IHR) Notification to PAHO/WHO
- IHR Assessment and Notification Process in Canada: Article 6 Notification
- Joint protocol to facilitate the reporting of food safety events to WHO via the IHR and INFOSAN networks
D4: Workforce development
- PHAC's Role in Building Public Health Workforce Capacity: Synthesis Report (2013)
- The Role of the Public Health Agency of Canada in Public Health Continuing Education: Informing a 5-Year Plan (March 2016)
- Public Health Workforce Development Strategy for the Public Health Agency of Canada Workforce (2015)
- Review of Pan-Canadian Framework for Public Health Human Resources Planning (Public Health Infrastructure Steering Committee, October 2016)
- Understanding the Role of Public Health Schools in Supporting a Skilled Public Health Workforce: A Report of the Meeting of the Canadian Network of Schools and Programs of Population and Public Health (March 2016)
R1: Preparedness
- Health Emergency Coordination Centre Standard Operating Procedures (British Columbia)
- Comprehensive Emergency Response Plan (British Columbia)
- Canada's International Emergency Response Framework
- Federal Policy for Emergency Management
- Federal Terrorism Response Plan
- Federal, Provincial and Territorial Public Health Response Plan for Biological Events
- Health Portfolio Emergency Response Plan
- Health Portfolio Mobilization Strategy for Event Response
- Health Portfolio Strategic Emergency Management Plan
- HPOC Concept of Operations
- HPOC Job Action Sheets
- HPOC Standard Operating Procedures
- National Emergency Response Framework.
- National Risk Profile for the Government of Canada.
- Operational Framework for Mutual Aid Requests
- Regional Emergency Coordination Centre Concept of Operations
- Regional Emergency Coordination Centre Job Action Sheets
- Regional Emergency Coordination Centre Standard Operating Procedures
R2: Emergency response operations
- Health Canada guide to risk characterization and reporting of chemical events
- Health Portfolio Emergency Response Plan
- Health Portfolio Emergency Response Structure
- Health Portfolio Mobilization Strategy for Event Response
- Health Portfolio Operations Centre Concept of Operations
- Health Portfolio Operations Centre Standard Operating Procedures
- Health Portfolio Operations Centre Job Action Sheets
- National Emergency Response Framework
- National Microbiology Laboratory standard operating procedures
- Regional Health Portfolio Emergency Response Plans
R3: Linking public health and security authorities
- Agreement on shared risk assessments between the Public Health Agency of Canada and the Canadian Food Inspection Agency
- Alberta Counter Terrorism Plan
- Avian Influenza Coordination Plan
- Memorandum of Understanding between RCMP and the Public Health Agency of Canada for provision of air transportation services by the RCMP to the Public Health Agency of Canada in the event of a national emergency
- Memorandum of Understanding between the Public Health Agency of Canada and Department of National Defence concerning the storage of smallpox vaccine and vaccinia immune globulin
- Memorandum of Understanding between the Public Health Agency of Canada and the Canadian Food Inspection Agency Concerning Food Safety on Passenger Conveyances and Ancillary Services
- Memorandum of Understanding between the Public Health Agency of Canada and the RCMP concerning the operations of the national CBRNE response team in response to the criminal or terrorist use of biological materials
- Memorandum of Understanding between the Public Health Agency of Canada, Health Canada and the Canadian Food Inspection Agency for Common Issues Related to Human Health
- Memorandum of Understanding between the Public Health Agency of Canada, Health Canada and Transport Canada Civil Aviation on the information exchange procedures dealing with any person, cargo or other thing on board an aircraft that could cause the spreading of a communicable disease
- Memorandum of understanding with the Royal Canadian Mounted Police regarding CBRNE response support
- Security Event Information Sharing Protocol
R4: Medical countermeasures and personnel deployment
- Agreement between the Government of Canada and the Government of the United States of America on Emergency Management Cooperation
- CONPLAN LASER
- CONPLAN RUBICON
- Operational Framework for Mutual Aid Request
- Health Portfolio Mobilization Strategy for Event Response
R5: Risk communication
- Health Canada and PHAC Toolkit for Health Risk Communications
- Health Emergency Risk Communications Protocol
- Health Portfolio Emergency Response Plan
- PHN Communicators Working Group Terms of Reference
- Protocols for risk communications
- Toolkit for Health Communicators
Points of entry
- Emergency Preparedness All Hazards Approach Manual (CBSA)
- Integrated Health Response Plans (British Columbia and Nova Scotia)
- Pandemic Guide (CBSA)
Chemical events
- Factsheet on the Operational Framework for Mutual Aid Requests
Radiation emergencies
- Canadian Guidelines for Intervention during a Nuclear Emergency (2003)
- Canadian Guidelines for the Restriction of Radioactively Contaminated Food and Water Following a Nuclear Emergency (2000)
- Generic Criteria and Operational Intervention Levels for Nuclear Emergency Planning and Response
Appendix 4: Tables and figures
Tables
- Table 1: Project Plan for Canada's Joint External Evaluation, June 2016 to June 2018
- Table 2: Canada's self-assessed indicator scores (National Forum, November 20-22, 2017)
- Table 3: Vaccination coverage reports and resources
- Table 4: Examples of provincial and territorial immunization campaigns
- Table 5: Canada's IHR reporting (data from January 1, 2011 to May 15, 2017)
Figures
- Figure 1: IHR National Focal Point organizational structure
- Figure 2: Coordination and information flow between National Focal Points and other sectors
- Figure 3: Oversight of pathogens and toxins and regulated plant pests in Canada
- Figure 4: Immunization delivery and information systems in Canada
- Figure 5: Flow diagram for reporting to INFOSAN and IHR network
- Figure 6: Governance structure for a coordinated response to a biological threat in Canada
- Figure 7: IHR designated points of entry in Canada